Graphene sponge helps lithium sulphur batteries reach new potential
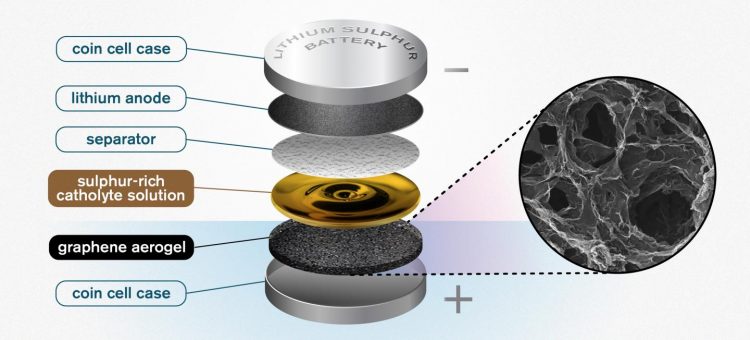
An illustration of the Chalmers design for a lithium sulfur battery. The highly porous quality of the graphene aerogel allows for high enough soaking of sulfur to make the catholyte concept worthwhile. Credit: Yen Strandqvist/Chalmers University of Technology
The researchers' novel idea is a porous, sponge-like aerogel, made of reduced graphene oxide, that acts as a free-standing electrode in the battery cell and allows for better and higher utilisation of sulphur.
A traditional battery consists of four parts. First, there are two supporting electrodes coated with an active substance, which are known as an anode and a cathode.
In between them is an electrolyte, generally a liquid, allowing ions to be transferred back and forth. The fourth component is a separator, which acts as a physical barrier, preventing contact between the two electrodes whilst still allowing the transfer of ions.
The researchers previously experimented with combining the cathode and electrolyte into one liquid, a so-called 'catholyte'. The concept can help save weight in the battery, as well as offer faster charging and better power capabilities. Now, with the development of the graphene aerogel, the concept has proved viable, offering some very promising results.
Taking a standard coin cell battery case, the researchers first insert a thin layer of the porous graphene aerogel.
“You take the aerogel, which is a long thin cylinder, and then you slice it – almost like a salami. You take that slice, and compress it, to fit into the battery,” says Carmen Cavallo of the Department of Physics at Chalmers, and lead researcher on the study. Then, a sulphur-rich solution – the catholyte – is added to the battery. The highly porous aerogel acts as the support, soaking up the solution like a sponge.
“The porous structure of the graphene aerogel is key. It soaks up a high amount of the catholyte, giving you high enough sulphur loading to make the catholyte concept worthwhile. This kind of semi-liquid catholyte is really essential here.
It allows the sulphur to cycle back and forth without any losses. It is not lost through dissolution – because it is already dissolved into the catholyte solution,” says Carmen Cavallo.
Some of the catholyte solution is applied to the separator as well, in order for it to fulfil its electrolyte role. This also maximises the sulphur content of the battery.
Most batteries currently in use, in everything from mobile phones to electric cars, are lithium-ion batteries. But this type of battery is nearing its limits, so new chemistries are becoming essential for applications with higher power requirements. Lithium sulphur batteries offer several advantages, including much higher energy density.
The best lithium ion batteries currently on the market operate at about 300 watt-hours per kg, with a theoretical maximum of around 350. Lithium sulphur batteries meanwhile, have a theoretical energy density of around 1000-1500 watt-hours per kg.
“Furthermore, sulphur is cheap, highly abundant, and much more environmentally friendly. Lithium sulphur batteries also have the advantage of not needing to contain any environmentally harmful fluorine, as is commonly found in lithium ion batteries,” says Aleksandar Matic, Professor at Chalmers Department of Physics, who leads the research group behind the paper.
The problem with lithium sulphur batteries so far has been their instability, and consequent low cycle life. Current versions degenerate fast and have a limited life span with an impractically low number of cycles. But in testing of their new prototype, the Chalmers researchers demonstrated an 85% capacity retention after 350 cycles.
The new design avoids the two main problems with degradation of lithium sulphur batteries – one, that the sulphur dissolves into the electrolyte and is lost, and two, a 'shuttling effect', whereby sulphur molecules migrate from the cathode to the anode. In this design, these undesirable issues can be drastically reduced.
Read the article, “A free-standing reduced graphene oxide aerogel as supporting electrode in a fluorine-free Li2S8 catholyte Li-S battery” published in the Journal of Power Sources.
A long journey to commercial potential
The researchers note, however, that there is still a long journey to go before the technology can achieve full market potential. “Since these batteries are produced in an alternative way from most normal batteries, new manufacturing processes will need to be developed to make them commercially viable,” says Aleksandar Matic.
More about the Chalmers labs used in this research
The researchers investigated the structure of the graphene aerogel at the Chalmers Materials Analysis Laboratory (CMAL). CMAL has advanced instruments for material research. The laboratory formally belongs to the Department of Physics, but is open to all researchers from universities, institutes and industry. The experiments in this study have been carried out using advanced and high-resolution electron microscopes.
Major investments, totalling around 66 million Swedish kronor have recently been made to further push CMAL to the forefront of material research.
The investments included the purchase of a monochromated and double aberration corrected (CETCOR image and ASCOR probe Cs-correctors) TEM JEOLARM (200 kV) 40-200, equipped with a field emission gun (FEG). This was the first paper to be published with the use of this brand-new microscope, which was used to investigate the structure of the aerogel.
The new microscope, which weighs as much as a full grown elephant, will be formally inaugurated on 15 May in a ceremony at Chalmers.
The Knut and Alice Wallenberg Foundation has contributed around half of the investments.
###
Read more about Chalmers Materials Analysis Laboratory here.
For more information, contact:
Carmen Cavallo
Researcher, Condensed Matter Physics, Department of Physics
Chalmers University of Technology
carmen.cavallo@chalmers.se
+46 31 772 33 10
Aleksandar Matic
Professor, Condensed Matter Physics, Department of Physics
Chalmers University of Technology
matic@chalmers.se
+46 31 772 51 76
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



