
Innovative Crop Engineering for a Growing Global Population
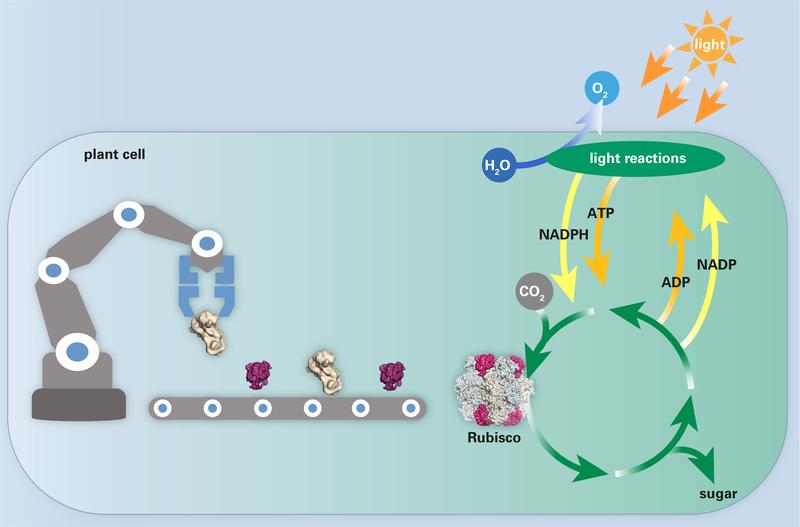
The Rubisco assembly line leads to the formation of the functional enzyme. Folding and assembly of the Rubisco subunits is assisted by chaperonins. Rubisco catalyses the key step of CO2 fixation.
The world's population is predicted to exceed 9 billion in 2050. With more mouths to feed, there is a pressing need for improved food output. To meet the global demand for food, scientists aim to increase the efficiency of photosynthesis and therefore crop productivity.
Boosting photosynthesis
Photosynthesis is the fundamental biological process that underlies all plant growth and supports life on Earth. Plants use the energy of sunlight to convert carbon dioxide (CO2) and water to sugar and oxygen (O2). The critical enzyme in this process is Rubisco. Rubisco catalyses the first step in carbohydrate production in plants, the fixation of CO2 from the atmosphere.
In doing so, plants utilize CO2 to build biomass and produce the required energy for growth. However, Rubisco is an inefficient enzyme as it captures CO2 slowly. Competing reactions with O2 further impair Rubisco’s catalytic efficiency. For these reasons, Rubisco often limits the rate of photosynthesis and ultimately plant growth, making Rubisco a hot target for genetic engineering.
Engineering of plant Rubisco, and photosynthesis, would be enhanced by functional expression of the enzyme in alternative hosts. So far, however, scientists failed to produce an enzymatically active form of plant Rubisco in a bacterial host – a goal that has been sought after for many decades. A team led by Manajit Hayer-Hartl, head of the research group “Chaperonin-assisted Protein Folding”, has now identified the requirements for expressing and assembling plant Rubisco in a bacterium. Their findings are expected to greatly accelerate efforts to improve photosynthesis through Rubisco engineering.
The Rubisco assembly line
The Rubisco enzyme consists of eight large and eight small subunits. Protein folding of the large subunits is assisted by specific chaperonins, macromolecular folding cages, in which the newly synthesized proteins can assume their proper functional conformation. After folding, multiple additional helper proteins (chaperones) assist in the proper assembly of the subunits into the large enzyme complex.
The researchers generated functional plant Rubisco in a bacterial host by simultaneously expressing plant chaperones and Rubisco in the same cells. This not only enables the scientists to understand the complex assembly pathway of Rubisco, but to modify the Rubisco gene in order to improve Rubisco’s properties. Once they have obtained a Rubisco variant with a desired trait, they can insert the modified gene back into the plant cells. This is a key-step towards improving photosynthesis through Rubisco engineering. “The bacterial expression system resembles an assembly line for cars. Whereas previously every optimized variant of Rubisco had to be painstakingly expressed in a transgenic plant, which takes a year or more to generate – like building a car by hand – we can now make hundreds or thousands of Rubisco variants in days or weeks. It is like building cars in an automated assembly line”, explains Hayer-Hartl.
Superior Rubisco variants
Genetic engineering facilitates efforts to generate Rubisco variants with improved functional properties. This might not only lead to the much-needed increase in crop yields, but also plant varieties with increased water-use efficiencies or enhanced temperature resistance – properties that are of special importance in the light of global warming and increasing water scarcity.
Originalpublikation
Aigner H*, Wilson RH*, Bracher A, Calisse L, Bhat JY, Hartl FU, Hayer-Hartl M. Plant Rubisco assembly in E. coli with five chloroplast chaperones including BSD2. Science, Dezember 2017. *These authors contributed equally to this work.
About Manajit Hayer-Hartl
Manajit Hayer-Hartl received her Bachelor of Science degree at the University of Stirling, Scotland, UK, where she afterwards gained her PhD. Her interest in structural and cellular biology motivated her to several postdoctoral fellowships at renowned research institutions, among them the Louis Pasteur Institute in Strasbourg, France and the Sloan-Kettering Institute in New York, USA. Hayer- Hartl joined the Max Planck Institute of Biochemistry in 1997 as group leader in the department “Cellular Biochemistry”. Since 2006, she is head of the research group “Chaperonin-assisted Protein Folding”. Her research focuses on chaperones and how these molecular machines assist in proper protein folding and assembly. Hayer-Hartl became EMBO Member in 2016 and was awarded the Dorothy Crowfoot Hodgkin Award in 2017.
About the Max Planck Institute of Biochemistry
The Max Planck Institute of Biochemistry (MPIB) belongs to the Max Planck Society, an independent, non-profit research organization dedicated to top level basic research. As one of the largest Institutes of the Max Planck Society, 850 employees from 45 nations work here in the field of life sciences. In currently eight departments and about 25 research groups, the scientists contribute to the newest findings in the areas of biochemistry, cell biology, structural biology, biophysics and molecular science. The MPIB in Munich-Martinsried is part of the local life-science-campus where two Max Planck Institutes, a Helmholtz Center, the Gene-Center, several bio-medical faculties of two Munich universities and several biotech-companies are located in close proximity. http://biochem.mpg.de
Contact:
Dr. Manajit Hayer-Hartl
Department of Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: mhartl@biochem.mpg.de
Dr. Christiane Menzfeld
Öffentlichkeitsarbeit
Max-Planck-Institut für Biochemie
Am Klopferspitz 18
82152 Martinsried
Tel. +49 89 8578-2824
E-Mail: pr@biochem.mpg.de
www.biochem.mpg.de
http://www.biochem.mpg.de/en – homepage max planck institute of biochemistry
http://www.biochem.mpg.de/en/rg/hayer-hartl – homepage Manajit Hayer-Hartl















