
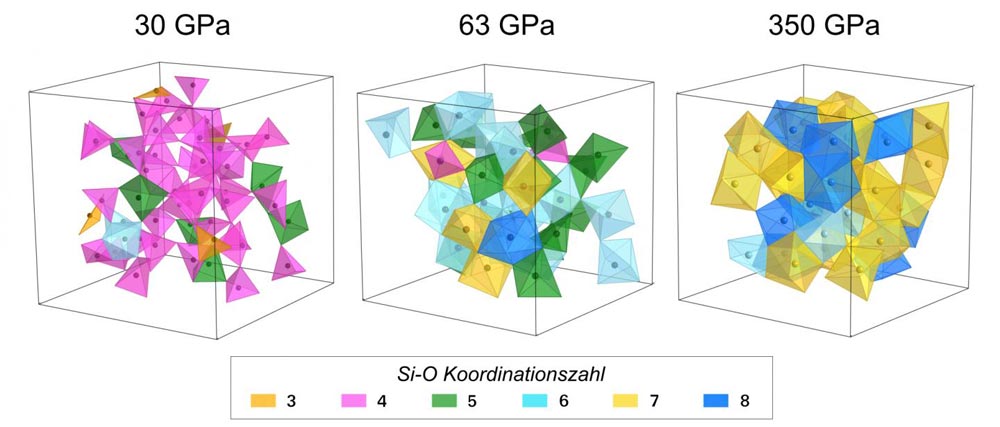
Using JSC's JUQUEEN supercomputer, University of Cologne researchers were able to simulate the structure of silicon dioxide at a variety of different pressures. The image shows how the the shape and structure of the atoms change as pressure increases.
Credit: Prescher, C., Prakapenka, V.B., Stefanski, J., Jahn, S., Skinner, L.B., Wang, Y.
In order to more fully comprehend the complexities of Earth's interior, humanity has to dig deep–literally. To date, scientists have been able to bore a little over 12 kilometres deep, or about half the average depth of the Earth's crust.
Why would researchers need to peer into deeper depths? Both to better understand how the earth formed and how the interior might have an effect on our life on the surface of the Earth today, such as by the magnitude and reversals of the Earth's magnetic field.
However, experiments investigating materials at conditions deep in the Earth are challenging, meaning that to continue gaining insights into these phenomena, experimentalists must turn to modeling and simulation to support and complement their efforts.
To that end, researchers at the University of Cologne's Institute for Geology and Mineralogy have turned to computing resources at the Jülich Supercomputing Centre (JSC) to help better comprehend how materials behave in the extreme conditions below the surface of the Earth.
The team, led by University of Cologne's Prof. Dr. Sandro Jahn and Dr. Clemens Prescher, has been using JSC's JUQUEEN supercomputer to simulate the structure of melts by studying silicate glasses as a model system for melts under ultra-high pressures. The team recently published its initial findings in the Proceedings of the National Academy of Sciences.
“Understanding properties of silicate melts and glasses at ultra-high pressure is crucial to understand how the Earth has formed in its infancy, where impacts of large asteroids led to a completely molten Earth,” said Prescher. “In fact, all of the internal layered structure we know today was formed in such events.”
It's a glass
When most people think of the word glass, they think of windows or bottles. Glass, however, is a term describing a wide range of non-crystal solids. Atoms in a solid can organize themselves in a variety of ways, and materials considered glasses have some of the more “chaotic” atomic structures possible in solids.
A glass can also be seen as a frozen melt. Thus by understanding the properties of glasses at ultra-high pressures, researchers can gain insights into the melts' properties in the deep Earth's interior, providing a clearer view into the physical processes which made the Earth and might be still occurring today.
Using a variety of geophysics measurements and laboratory experiments, researchers are capable of gaining some degree of insight into material properties under certain pressure conditions without actually being able to make direct observations.
Enter supercomputing. As computing power has gotten stronger, geophysics researchers are able to complement and expand their studies of these inner-Earth processes through the use of numerical models.
In the case of the University of Cologne researchers, they wanted to get a more detailed insight into the structure of the silicate glass than their experimental efforts were able to provide. The team utilized ab initio calculations of atoms' electronic structures and put these calculations in motion using molecular dynamics simulations. Ab initio calculations mean that researchers start with no assumptions in their mathematical models, making a simulation more computationally expensive but also more accurate.
Due to having many calculations for each atom's structure and computationally demanding molecular dynamics calculations, the team keeps its simulations relatively small in scale–the team's largest runs typically have between 200-250 atoms in the simulation. This size allows the team to run simulations under a variety of different pressure and temperature combinations, ultimately allowing it to calculate a small but representative sample of material interactions under a variety of conditions.
To test its model and lay the foundation for modeling increasingly complex material interactions, the team decided to simulate silicon dioxide (SiO2), a common, well-studied material, most well-known as the compound that forms quartz.
Among silicate materials, SiO2 is a good candidate on which to base computational models–researchers already understand how its atomic structure patterns and material properties change under a variety of pressure conditions.
The team chose to focus on a relatively simple, well-known material in order to expand the range of pressure it could simulate and attempt to validate the model with experimental data. Using JUQUEEN, the team was able to extend its investigation well beyond the experimentally achieved 172 Gigapascals, corresponding to 1.72 million times the Earth's atmospheric pressure, or roughly the amount of pressure the Eiffel Tower would apply by pressing down on the tip of a person's finger.
The researchers also found that at high pressures, oxygen atoms are much more compressible than silicon atoms. The varying size ratio between the two leads to hugely different glass structures of SiO2 at low and at high pressures.
Digging Deeper
By validating its model, the team feels confident that it can move on to more complex materials and interactions. Specifically, the team hopes to expand its investigations deeper into the realm of melts. Think of lava as a melt–molten rock erupts from below the earth's surface, rapidly cools when it reaches the surface, and may form obsidian, a glassy rock.
In order to do more advanced simulations of melts, the team would like to be able to expand its simulations to account for a wider range of chemical processes as well as expand the number of atoms in a typical run.
As JSC and the other two Gauss Centre for Supercomputing (GCS) facilities–the High-Performance Computing Center Stuttgart and the Leibniz Supercomputing Centre in Garching–install next-generation supercomputers, the team is confident that they will be able to gain even greater insight into the wide range of complex material interactions happening many kilometres below the surface.
“A faster machine will enable us to simulate more complex melts and glasses, which is crucial to go from model systems, such as SiO2 glass in this study, to the real-world compositions we expect in the Earth's interior,” Prescher said.
Prescher also noted that JSC support staff helped the team work more efficiently by assisting with implementing the team's code.
This type of support represents GCS' plans for the future. With the promise and opportunity connected to next-generation computing architectures, GCS centre leadership realizes that closer collaboration with users and application co-design will be a key component for ensuring researchers can efficiently solve bigger, more complex scientific problems.
Whether studying deep in space among the stars or deep below the surface of the Earth, the collaboration between supercomputing centres and researchers will play an increasingly important role in solving the world's toughest scientific challenges.
###
This research used Gauss Centre for Supercomputing resources based at the Jülich Supercomputing Centre.












