Self-repairing batteries
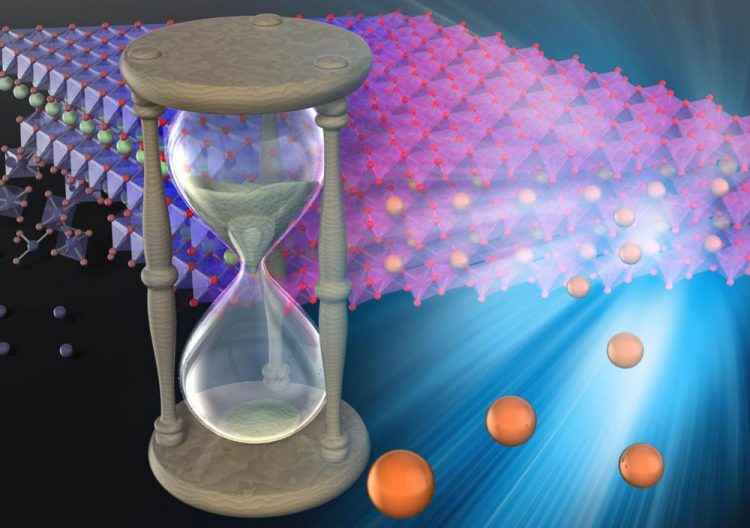
Self-repairing batteries would have longer lifetimes than batteries at present. Credit: © 2019 Atsuo Yamada
Engineers at the University of Tokyo continually pioneer new ways to improve battery technology. Professor Atsuo Yamada and his team recently developed a material which could significantly extend the life of batteries and afford them higher capacities as well.
From smartphones to pacemakers and now even cars, batteries power much of our world and their importance only continues to grow. There are two particular aspects of batteries that many believe need to improve to meet our future needs. These are the longevity of the battery and also its capacity – how much charge it can store.
The chances are your devices use a type of battery called a lithium-ion battery. But another kind based on sodium rather than lithium may become commonplace soon.
Both kinds of battery can store and deliver a large amount of charge, thanks to the way constituent materials pass electrons around. But in both lithium and in sodium batteries, repeated cycles of charging and usage can significantly reduce the storage capacity over time.
If you could see inside a typical battery, you would see layers of metallic material. As batteries charge and discharge, these layers degrade and develop cracks or flakes – called stacking faults – which reduce the batteries' ability to store and deliver charge.
These stacking faults occur because the material is held together by a weak force called the Van der Waals force, which is easily overwhelmed by the stress put on the materials during charging and use.
Yamada and colleagues demonstrated that if the battery is made with a model material – oxygen redox-layered oxide (Na2RuO3) – then something remarkable happens. Not only does the degradation from charge and discharge cycles diminish, but the layers actually self-repair.
This is because the material the researchers demonstrated is held fast by a force called coulombic attraction, which is far stronger than the Van der Waals force.
“This means batteries could have far longer life spans, but also they could be pushed beyond levels that currently damage them,” said Yamada. “Increasing the energy density of batteries is of paramount importance to realize electrified transportation.”
###
Journal article
Benoit Mortemard de Boisse, Marine Reynaud, Jiangtao Ma, Jun Kikkawa, Shin-ichi Nishimura, Montse Casas-Cabanas, Claude Delmas, Masashi Okubo and Atsuo Yamada. Coulombic self-ordering upon charging large-capacity layered battery electrodes. Nature Communications. DOI: 10.1038/s41467-019-09409-1
Supported by a JSPS Grant-in-Aid for Specially Promoted Research (No. 15H05701).
Related links
Yamada & Okubo Laboratory – http://yamada-lab.
Graduate School of Engineering – http://www.
Research Contact
Professor Atsuo Yamada
Department of Chemical System Engineering, Graduate School of Engineering,
The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 JAPAN
Tel: +81-3-5841-7295
Email: yamada@chemsys.t.u-tokyo.ac.jp
Press Contact
Mr. Rohan Mehra
Division for Strategic Public Relations, The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8654 JAPAN
Tel: +81-3-5841-0876
Email: press-releases.adm@gs.mail.u-tokyo.ac.jp
About the University of Tokyo
The University of Tokyo is Japan's leading university and one of the world's top research universities. The vast research output of some 6,000 researchers is published in the world's top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 2,000 international students. Find out more at https:/
Media Contact
More Information:
http://dx.doi.org/10.1038/s41467-019-09409-1All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

New organoid with all key pancreas cells
Researchers from the Organoid group (previously Clevers group) at the Hubrecht Institute have developed a new organoid that mimics the human fetal pancreas, offering a clearer view of its early development….

Unlocking the potential of nickel
New study reveals how to use single atoms to turn CO2 into valuable chemical resources. Nickel and nitrogen co-doped carbon (Ni-N-C) catalysts have shown exceptional performance in converting CO2 into…

‘Spooky action’ at a very short distance
Scientists map out quantum entanglement in protons. Particles streaming from collisions offer insight into dynamic interactions and collective behavior of quarks and gluons. Scientists at the U.S. Department of Energy’s…



