Chemists develope a new method for the formation of fluorinated molecular rings
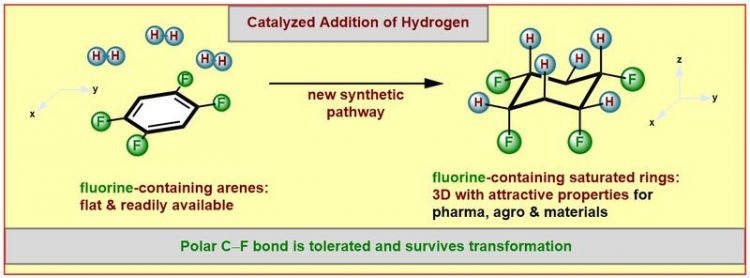
Illustration of the new synthetic method WWU/Frank Glorius
Dyes, pharmaceuticals, and functional materials – all of these products are generally based on innovative molecules made by chemists. For their production several chemical reactions are available to the expert, however, limitations remain. For example, fluorinated compounds, molecules that contain at least one fluorine atom, are often rather difficult to prepare.
This is unfortunate, since they exhibit interesting chemical properties and are of greatest importance for the development of active ingredients. Thus, new ways have to be found to produce these compounds. Now, chemists from the Westfälische Wilhems-Universität (WWU) made the impossible possible: they have developed a new and practical synthetic method for the formation of such fluorinated three-dimensional “saturated” (meaning only single-bond containing) molecular ring structures.
The report of Prof. Dr. Frank Glorius, Mario Wiesenfeldt, Dr. Zackaria Nairoukh and Dr. Wei Li has just been published online in the learned journal “Science“.
“I feel that our results are a breakthrough. It can have great importance for the efficient production of new molecules and, consequently, new drugs, crop protection agents and functional materials” is the assessment of Frank Glorius.
His new synthetic method starts from flat, “aromatic“ (and thus very stable) ring structures built up from carbon and bearing fluorine atoms. These starting materials employed by the Münster scientists are either low priced commercially available compounds or they can be readily made.
Facilitated by a catalyst the chemists succeeded to add hydrogen atoms (“hydrogenation”) selectively to one face of the ring system. Chemists and biochemists define catalysts as enzymes or molecules that can speed up or enable certain reactions. A selective addition allows the control of the properties of the products formed, for example the solubility, the aggregate state or the polarity. A molecule is considered to be “polar”, if charges are separated to result in more negative and more positive molecular fragments. The products formed in this study contain the more negatively charged fluorine atoms on one face and the more positively charged hydrogen atoms on the other face of the ring.
Many different fluorinated aromatic starting materials were successfully converted into the desired products by the group of Frank Glorius. “For two reasons the success of our work was rather unexpected” stresses Frank Glorius. “The attached fluorine atoms reduce the reactivity of the already not very reactive aromatic starting materials in the catalytic hydrogenation even further. This is especially true for substrates containing multiple fluorine atoms. Even more pronounced is the sensitivity of the carbon-fluorine bond against hydrogenation, generally leading to the loss of the fluorine atom.”
Many studies of the past had observed this latter problem. Remarkably, the new synthetic method allows fluorine atoms to tolerate the catalytic hydrogenation. “We have identified a catalyst system that is powerful enough to overcome the aromatic stabilization. Yet it is mild enough to preserve the carbon-fluorine bonds.” As a catalyst the scientists from Münster utilize a combination of the noble metal rhodium and an especially electron-rich carbene-ligand (a special “metal-binding” molecule) that greatly influences the properties of the catalyst.
First author Mario Wiesenfeldt summarizes: “The new method provides surprisingly simple access to a fascinating structural motif: cyclic, saturated and selectively fluorinated on one face. Many of the products are characterized by a high level of polarity.”
Easily prepared in one step and in larger amounts
Some background information: The compound “all-cis-1,2,3,4,5,6-hexafluorocyclohexane“, in which the saturated six-membered carbon-cycle contains the maximum number of 6 fluorine atoms on the same face of the ring, represents one of the most polar organic molecules known to date. Only in 2015 this remarkable compound was first prepared and reported by Prof. David O’Hagan from the University of St. Andrews in Scotland. However, his team required a twelve-step synthetic sequence for its formation. The new method allows the formation of this and many related compounds in a convenient single step, thus allowing the formation of larger amounts.
Asymmetric hydrogenation of arenes as a remaining challenge
“Hydrogenation is an attractive and often very clean method of synthesis“ stresses Frank Glorius. “An especially prominent example is the formation of ammonia through the Haber-Bosch process, the hydrogenation of nitrogen, consuming more than 1% of the world's annual energy supply. It is of fundamental importance for the nutrition of mankind, since it serves as a basis for the production of nitrogen fertilizer, among others.“ In addition the importance is also reflected by the three Nobel prizes given for this topic (Fritz Haber 1918, Carl Bosch 1931, Gerhard Ertl 2007). Also important is the hydrogenation of organic compounds, last decorated with a Nobel price for the asymmetric hydrogenation of aromatic compounds in 2001 (William S. Knowles und Ryoji Noyori). Chemoselective and asymmetric hydrogenation reactions of aromatic compounds remain to be challenging.
This work was financed by the Studienstiftung des Deutschen Volkes (Mario P. Wiesenfeldt), the Hans Jensen Minerva Foundation (Zackaria Nairoukh), the Alexander von Humboldt Foundation (Wei Li) and the Deutsche Forschungsgemeinschaft (Gottfried Wilhelm Leibniz Prize for Frank Glorius). Based on these results a patent application was filed.
Original publication:
Mario P. Wiesenfeldt, Zackaria Nairoukh, Wie Li und Frank Glorius: Hydrogenation of fluoroarenes: Direct access to all-cis-(multi)fluorinated cycloalkanes. Science; Published online 10 Aug 2017; DOI: 10.1126/science.aao0270
http://science.sciencemag.org/content/early/2017/08/09/science.aao0270 Original publication in “Science”
https://www.uni-muenster.de/Chemie.oc/glorius/ Glorius group
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



