
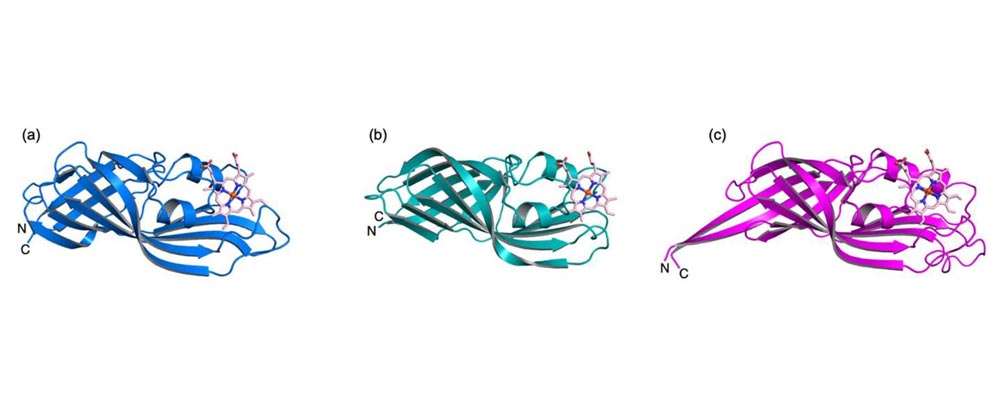
Figure 1. Crystal structures of (a) N-terminal domain of HtaA, (b) C-terminal domain of HtaA, and (c) HtaB.
Credit: NINS/IMS
Research Background:
Iron ion is an essential trace element for all organisms. The organisms utilize the various molecular systems for uptake iron ions into their cells.
Pathogenic bacteria have a molecular system for extracting heme from heme proteins in infected hosts, by which extracted heme molecules are transported into the bacterial cells so that they utilize heme proteins in infected hosts as the major iron source.
Before this study, the heme uptake systems from several bacteria have been studied, but the detail studies of the heme uptake system from Corynebacteria including Corynebacterium diphtheriae has not progressed.
Results:
The heme uptake system from Corynebacteria consists of HtaA and HtaB, which are located on the cell surface and are responsible for heme binding and transport, and HmuT-HmuU-HmuV, which is responsible for heme transport into the cell. HtaA extracts heme from the host heme protein and transfers extracted heme to HtaB. HtaB transfers heme to HmuT. At last, heme was transported into the cell by HmuUV heme transporter.
Our research group has previously reported the crystal structure of HmuT from Corynebacterium glutamicum (Muraki et al., Chem. Lett., 2016, Muraki et al., Int. J. Mol. Sci., 2016). In this study, we have succeeded to determine the crystal structures of HtaA and HtaB to find that HtaA and HtaB have a novel fold for heme-binding/transport proteins.
HtaA consists of an N-terminal domain and a C-terminal domain, in which the CR (conserved region) domain is adopted for heme-binding/transport. HtaB consists of a single domain. We determined the crystal structures of the N- and the C-terminal domains of HtaA and HtaB at 2.0 Å, 1.3 Å and 1.7 Å resolution, respectively. These structures are homologous to each other, and consist of eleven β-strands, which formed antiparallel β-sheets and two short α-helices. A part of antiparallel β-sheets formed a barrel-like structure (Fig. 1).
We have determined the crystal structures of HtaA/HtaB in the holo-form. One heme molecule is bound in the heme pocket formed by the loop region at the end of β-sheet and α-helix (α1). The propionate group of heme forms hydrogen bonds with serine (Ser54 in HtaA) and tyrosine (Tyr201 in HtaA). The ring of heme was stabilized by a π-π interaction with phenylalanine (Phe200 in HtaA). Heme Iron had a five-coordinate structure with tyrosine (Tyr58 in HtaA) (Fig. 2). This tyrosine forms a hydrogen bond with histidine (His111 in HtaA). These amino acids involved in heme recognition are highly conserved between HtaA / HtaB CR domains.
We have found that the hydrogen bond between the axial ligand Tyr and His plays an important role for the regulation of heme-binding affinity fo HtaA/HtaB. By replacing this His with Ala, we prepared an apo-protein, and determined the apo-form structure of the C-terminal domain of HtaA. The apo form of HtaA forms a domain swapped dimer (Fig. 3). In this structure, α-helix (α1) in one protomer is located close to the heme pocket of the other protomer. This domain-swapped dimer seems to mimic the reaction intermediate of the heme transfer reaction in HtaA/HtaB.
Social significance of this research:
In this study, we have determined the crystal structures of the heme uptake system from Corynebacterium glutamicum, which is a non-pathogenic bacterium, but the same heme uptake system is also used by Corynebacterium diphtheriae. The results of this research may contribute to the development of new antibiotics that inhibit the growth of Corynebacterium diphtheriae by blocking the uptake of iron ions (heme) essential for growth.
###












