Intestinal inflammation: immune cells protect nerve cells after infection
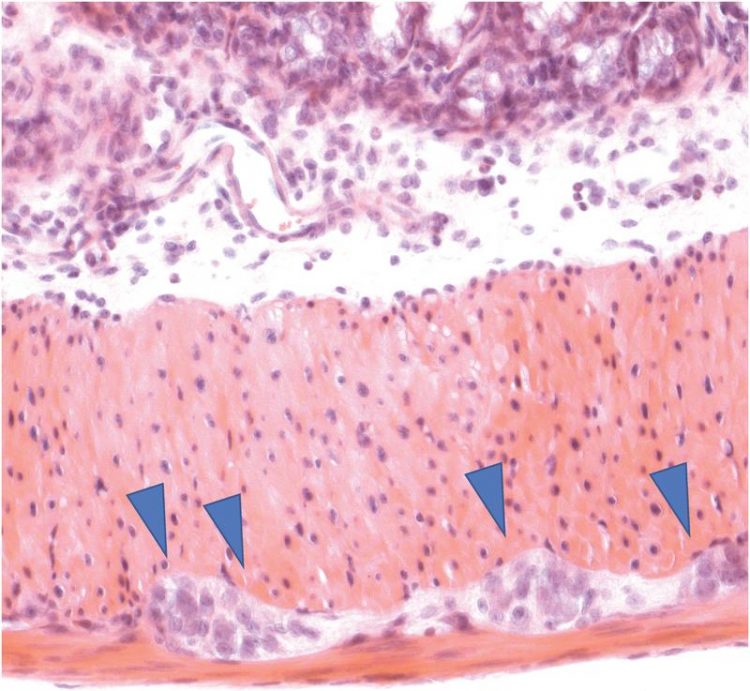
The gut is permeated by nerve cells (embedded in muscle tissue, marked by blue arrows). They form a separate nervous system that for example regulates gastrointestinal motility. P. Rosenstiel, IKMB Kiel University.
In the event of an infection in the gastrointestinal tract, part of the tissue becomes inflamed, and intestinal nerve cells die off. Among other things, this loss in turn often leads to a restriction of the gastrointestinal motility, which is essential for a healthy digestion.
In many cases, this is also followed by additional diseases, such as the so-called irritable bowel syndrome.
The reason for the nerve cells dying off has previously been unclear. An international research team involving Professor Philip Rosenstiel, Director at the Institute of Clinical Molecular Biology (IKMB) at Kiel University (CAU), has now succeeded in describing the mechanism that leads to the death of the nerve cells.
Specific immune cells in the gut appear to protect the nerve cells, as the team was able to demonstrate in their current research. The scientists, led by Daniel Mucida, Associate Professor at The Rockefeller University in New York, recently published their results in the renowned scientific journal Cell.
The human gastrointestinal tract is permeated by nerve cells, which form a separate enteric nervous system. This controls digestion largely independently, for example by regulating the gastrointestinal motility. Previous studies have shown that the immune system in the gut also plays an important role.
For example, specific immune cells regulate the activity of the nerve cells in healthy individuals. These interactions between nerve and immune cells are disrupted by diseases such as chronic inflammatory bowel disease or irritable bowel syndrome.
“The research shows that immune cells are also important for the survival of nerve cells in the case of infections,” said Professor Philip Rosenstiel, member of the Steering Committee of the Cluster of Excellence “Precision Medicine in Chronic Inflammation” (PMI). Accordingly, specific immune cells recognize the infection and limit the damage to nerve cells.
The specific cells are so-called macrophages in the gastrointestinal muscular layer. These are scavenger cells which detect and destroy pathogens. In the event of an infection, these macrophages initiate a protection program, in which they produce certain substances that ultimately protect the nerve cells.
In turn, this protection program is apparently dependent on the microorganisms living in the intestine, the so-called microbiome. If these are absent, or if their balance is disturbed, the nerve cells are also no longer protected.
“The so-called gut-brain axis, through which the intestinal microbiome, the intestinal cells and the central nervous system cooperate, is an important topic for the future at the Cluster of Excellence PMI. Because the gut-brain axis could lead to new approaches for treating chronic inflammation, and also neurological diseases,” said Professor Rosenstiel.
“The mechanism, which has now been discovered in cooperation with Daniel Mucida and his team from New York, through which the immune system in the gut protects nerve cells in the event of an infection, is an important building block in the exploration of the gut-brain axis. We want to expand on this at the Cluster of Excellence, and increase our research efforts in this area in future,” added Rosenstiel.
Photos are available to download:
https://precisionmedicine.de/pm/material/2020_Darmnervenzellen.pdf
The gut is permeated by nerve cells (embedded in muscle tissue, marked by blue arrows). They form a separate nervous system that for example regulates gastrointestinal motility, which is essential for a healthy digestion.
Photo: P. Rosenstiel, IKMB Kiel University.
https://precisionmedicine.de/pm/material/2020_Rosenstiel.jpg
Prof. Dr Philip Rosenstiel, Steering Committee-member of the Cluster of Excellence “Precision Medicine in Chronic Inflammation” (PMI) and Director at the Institute of Clinical Molecular Biology, CAU, UKSH.
Photo/Copyright: T. Böschen / Cluster of Excellence PMI, Uni Kiel.
The Cluster of Excellence “Precision Medicine in Chronic Inflammation” (PMI) is being funded from 2019 to 2025 through the German Excellence Strategy (ExStra). It succeeds the “Inflammation at Interfaces” Cluster, which was already funded in two periods of the Excellence Initiative (2007-2018). Around 300 members from eight institutions at four locations are involved: Kiel (Kiel University, University Medical Center Schleswig-Holstein (UKSH), Muthesius University of Fine Arts and Design, Kiel Institute for the World Economy (IfW), Leibniz Institute for Science and Mathematics Education (IPN)), Lübeck (University of Lübeck, University Medical Center Schleswig-Holstein (UKSH)), Plön (Max Planck Institute for Evolutionary Biology) and Borstel (Research Center Borstel – Leibniz Lung Center).
The goal is to translate interdisciplinary research findings on chronic inflammatory diseases of barrier organs to healthcare more intensively, as well as to fulfil previously unsatisfied needs of the patients. Three points are important in the context of successful treatment, and are therefore at the heart of PMI research: the early detection of chronic inflammatory diseases, the prediction of disease progression and complications, and the prediction of individual responses to treatment.
Cluster of Excellence “Precision Medicine in Chronic Inflammation”
Scientific Office, Head: Dr habil. Susanne Holstein
Postal address: Christian-Albrechts-Platz 4, 24118 Kiel, Germany
Contact: Sonja Petermann
Tel.: +49 431 880-4850, Fax: +49 (0)431 880-4894
E-mail: spetermann@uv.uni-kiel.de
Twitter: PMI @medinflame
Press contact:
Frederike Buhse
Tel.: +49 (0)431 880 4682 e-mail: fbuhse@uv.uni-kiel.de
https://precisionmedicine.de/
Prof. Dr Philip Rosenstiel
Institute of Clinical Molecular Biology,
Kiel University (CAU), University Medical Center Schleswig-Holstein (UKSH)
Tel.: +49 (0)431 500 15111
p.rosenstiel@mucosa.de
Fanny Matheis, Paul A. Muller, Christina L Graves, Ilana Gabanyi, Zachary J. Kerner, Diego Costa-Borges, Thomasz Ahrends, Philip Rosenstiel, Daniel Mucida: Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell (2020). doi.org/10.1016/j.cell.2019.12.002
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

New organoid with all key pancreas cells
Researchers from the Organoid group (previously Clevers group) at the Hubrecht Institute have developed a new organoid that mimics the human fetal pancreas, offering a clearer view of its early development….

Unlocking the potential of nickel
New study reveals how to use single atoms to turn CO2 into valuable chemical resources. Nickel and nitrogen co-doped carbon (Ni-N-C) catalysts have shown exceptional performance in converting CO2 into…

‘Spooky action’ at a very short distance
Scientists map out quantum entanglement in protons. Particles streaming from collisions offer insight into dynamic interactions and collective behavior of quarks and gluons. Scientists at the U.S. Department of Energy’s…



