
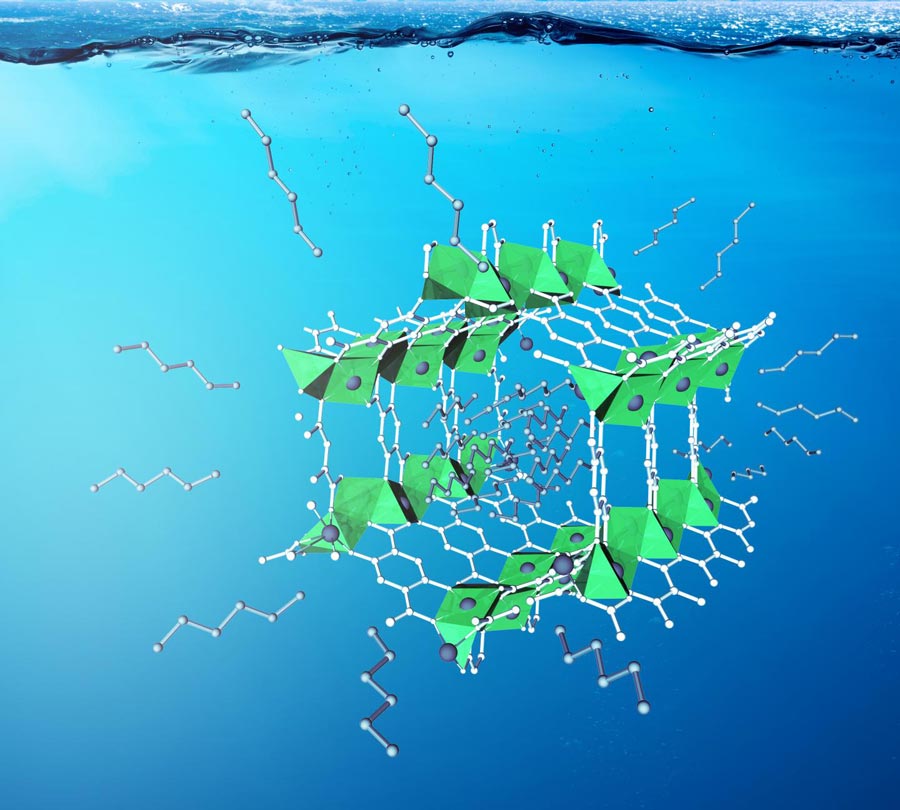
Micropores of MOFs with certain topologies increase the density of the olefins while partially preventing the adsorption of the synthesis gas.
Credit: @PSI
Efforts to develop heterogeneous catalysts that appeal to the fine chemical industry have been limited by underwhelming results. Although some approaches have shown promising catalytic activity, “heterogenization” itself is not enough.
To be adopted by industry, heterogeneous catalysts must promote selectivity that is difficult or even impossible to obtain with existing catalytic systems–the chemical properties of any proposed heterogeneous catalysts must go beyond easier separation and recycling.
The chemical-flexibility, tuneable pore size and chemical and structural stability of metal-organic frameworks (MOFs) makes them ideal for designing active sites at the molecular level. Able to selectively adsorb different molecules depending on their structure, they can direct selectivity and reaction performance.
Many promising catalytic applications using MOFs as precursors for novel materials as well as model systems for understanding heterogeneous catalysis processes have been described. The field of catalysis by MOFs is still in its infancy though since most examples are proof-of-concepts and do not offer attractive advantages to existing catalysts.
In the paper Metal-organic frameworks as kinetic modulators for branched selectivity in hydroformylation, researchers from the Paul Scherrer Institute's Syncat Group, led by Marco Ranocchiari, and EPFL's Laboratory of Molecular Simulation, a computational group led by Berend Smit, used the example of hydroformylation to show how adsorption properties of MOFs can be exploited in catalysis to get results that have otherwise been inaccessible.
The methods presented can be used to predict the effect of such microporous co-catalysts in increasing selectivity in any homogeneous or heterogeneous catalytic reaction.
Hydroformylation, or oxo synthesis, is an industrial process for obtaining aldehydes from olefins. Current catalytic processes yield both linear aldehydes, which are key intermediates for the detergent and polymer industry, and branched ones, which are considered a powerful tool for the fine chemical industry because of their possible use in producing enantioenriched products, that is, products featuring a greater proportion of a given enantiomer of a chiral substance.
The linear isomers are often formed with rhodium catalysts. Branched aldehydes are formed from rhodium catalysts with bidentate ligands with directing groups to enhance selectivity. Producing the sought-after branched isomers without these directing groups is still a challenge though and can only be achieved through complex Rh catalysts.
They have been shown to result, for instance, in a selectivity for 2-methylhexanale from 1-hexene up to 75% and up to 86% for 2-methylbutanale from 1-butene.
The researchers first screened several catalytic conditions to maximize the yield of the branched product that could be obtained with homogeneous catalysis. They then showed how they could go beyond this limit and achieve much higher branched selectivity by adding MOFs to the reaction mixture. They also tested different MOF topologies to understand the role of the MOF environment in such a change in selectivity.
The group was able to show that the micropores of MOFs push the cobalt-catalyzed hydroformylation of olefins to kinetic regimes that favor high branched selectivity, without the use of any directing groups. The addition of MOFs allowed branched selectivity of up to 90% in these cases, a feat that cannot be achieved with existing catalysts.
Monte Carlo and density functional theory simulations combined with kinetic models show that the micropores of MOFs with certain topologies increase the density of the olefins while partially preventing the adsorption of the synthesis gas–this is what leads to the high branched selectivity.
Though the research focused on aldehydes, the methods presented can be used to predict the effect of microporous co-catalysts in increasing selectivity in any homogeneous or heterogeneous catalytic reaction.
Researchers can determine the microporous material that has the best chances of increasing selectivity by first choosing those that can adsorb the catalyst while being inert under reaction conditions, and by then using simulations to determine how the microporous materials might change the local concentration of the selectivity determining reactant(s) within the micropores.












