
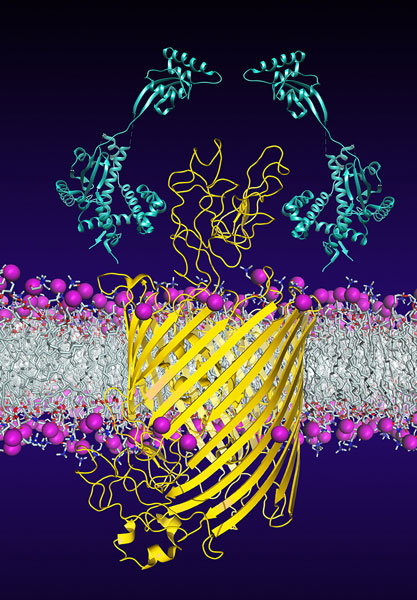
Chaperones (light blue) promote the insertion and folding of the bacterial membrane protein FhuA (yellow).
University of Basel, Biozentrum
Cellular machines continuously produce long polypeptide chains, the proteins. In order to properly fulfill its cellular function, a protein must however first adopt its correct spatial structure. In each cell there are molecular helper proteins called chaperones. They take care of the immature proteins to help them in the folding process and thus preventing errors.
The scientists led by Prof. Sebastian Hiller from the Biozentrum, University of Basel, and Prof. Daniel Müller from the Department of Biosystems Science and Engineering (D-BSSE), ETH Zurich in Basel have discovered how two chaperones in the gut bacterium E. coli protect the membrane protein FhuA during transport and assist its insertion into the membrane.
Chaperones help insertion of membrane protein
Countless proteins, which transport nutrients and signaling molecules, are embedded in the outer membrane of bacteria. One of these membrane transporters is the protein FhuA. Via this protein, the bacteria take up vitally important iron but also antibiotics. But how does the very large, barrel-shaped FhuA protein reach the outer membrane intact? The scientists from the Biozentrum and the D-BSSE have investigated this process more deeply.
In order to reach its goal in the outer membrane, FhuA uses the help of several chaperones. Using structural analyses and single-molecule force spectroscopy, the researchers have now elucidated how these two chaperones stabilize the immature protein and prevent misfolding. “This process is extremely dynamic,” says Hiller.
“Under the protection of the chaperones, within a millisecond, FhuA constantly changes its structure. It thus explores energetically favorable conformations which enable the stepwise insertion and folding of individual protein segments into the membrane.” With the insertion of the final protein segment, FhuA acquires its mature and functional barrel structure. Left unprotected, FhuA would fold incorrectly and finally aggregate.
Protein chaos without chaperones
Chaperones are significantly involved in the formation of functional proteins. They play an important role in the correct folding of soluble proteins and furthermore are necessary for the insertion of membrane proteins into the bacterial outer membrane. Because several organelles in plant and animal cells are of bacterial origin, chaperones also protect their membrane proteins in a similar manner and assist during membrane insertion. The new findings are consequently of great relevance also for diseases caused by misfolded proteins such as Alzheimer's, Parkinson's or cystic fibrosis.
“It has been known for a long time that chaperones protect other proteins from misfolding and encourage them to fold correctly. Now, our work has succeeded in demonstrating – for the first time in biological membranes – how chaperones support the membrane proteins that are key to pharmaceutical research,” explains ETH Professor Daniel Müller. Until recently, these could almost only be investigated using artificial environments. However, this meant that there was barely any understanding of how proteins fold into a cell’s membrane.
“To give a loose analogy, until now it was like putting a cow on a sheet of ice in order to investigate its natural behaviour and then observing surprising reactions,” says Müller. “We now have a better understanding of how the cell incorporates its molecular machines into membranes so that they can perform their versatile duties.”
Original source
Johannes Thoma, Björn M Burmann, Sebastian Hiller & Daniel J Müller
Impact of holdase chaperones Skp and SurA on the folding of β-barrel outer-membrane proteins
Nature Structural & Molecular Biology (2015), doi: 10.1038/nsmb.3087
Further information
Prof. Sebastian Hiller, University of Basel, Biozentrum, tel. +41 61 267 20 82, email: sebastian.hiller@unibas.ch
Prof. Daniel J. Müller, ETH Zurich, Department of Biosystems Science and Engineering, tel. +41 61 387 33 07, email: daniel.mueller@bsse.ethz.ch












