
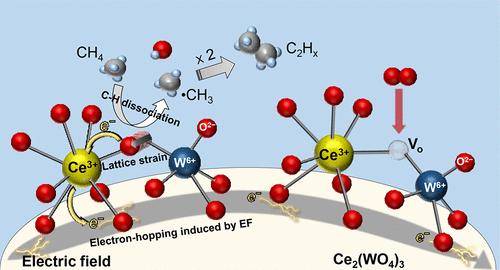
Reaction mechanisms for the oxidative coupling of methane (OCM) over Ce2(WO4)3 catalysts at low temperatures in an electric field.
Credit: Waseda University
The increased supply and optimized cost of natural gas have pushed chemical industries to actively seek for new ways of converting methane, the main constituent of natural gas, to ethylene, a hydrocarbon widely used in chemical products such as plastic.
Oxidative coupling of methane (OCM) is of high interest as a potentially efficient method but has yet to become commercially practical for various reasons, for example, the reaction temperature of over 700ºC requiring expensive equipment with high heat resistance, increasing production cost.
Scientists at Waseda University discovered a new reaction mechanism of performing OCM at a temperature as low as 150ºC. The novel catalytic reaction found in the study, which demonstrated both high yield and catalytic activity, was done in an electric field, and could provide a more cost-effective method of synthesizing ethylene in the future.
Their findings were published online in the Journal of Physical Chemistry C on January 22, 2018.
“Performing OCM in an electric field dramatically lowered the reaction temperature, and we succeeded in efficiently synthesizing C2 hydrocarbon, including ethylene, from oxygen in the atmosphere with methane,” says Shuhei Ogo, assistant professor of catalytic chemistry at Waseda.
He explains that by applying an electric field, the lattice oxygen of a catalyst is activated and becomes a reactive oxygen species even at low temperature, such as 150ºC. “The redox reaction mechanism, which repeats the consumption and regeneration of the reactive oxygen species on the catalyst surface, keeps the catalytic reaction cycle going.” Reports on such phenomenon are unprecedented, and the results of this study are considered to be the first of its kind in the world.
This reaction mechanism can lower the production cost of ethylene because high-temperature heat sources and large-scale heat exchanger devices become unnecessary, holding the cost on facilities and its management down as well. Not only large-scale manufacturers, but small to mid-sized gas wells with smaller production scales can also benefit from the reduced cost.
“The findings of this study can be utilized for various kinds of catalytic reaction that proceeds by redox reaction mechanism, while providing high selectivity and stability as well as energy efficiency at low temperature,” Ogo adds.
The research group plans to further investigate the highly active and selective catalyst in the electric field.
###
Reference
Published online in the Journal of Physical Chemistry C on January 22, 2018
Electron-hopping brings lattice strain and high catalytic activity in low temperature oxidative coupling of methane in an electric field
Authors: Shuhei Ogo*, Hideaki Nakatsubo, Kousei Iwasaki, Ayaka Sato, Kota Murakami, Tomohiro Yabe, Atsushi Ishikawa, Hiromi Nakai, and Yasushi Sekine
*Corresponding author: ogo@aoni.waseda.jp DOI: 10.1021/acs.jpcc.7b08994
University news on this research
About Waseda University
Waseda University is a leading private, non-profit institution of higher education based in central Tokyo, with over 50,000 students in 13 undergraduate and 20 graduate schools. Founded in 1882, Waseda cherishes three guiding principles: academic independence, practical innovation and the education of enlightened citizens. Established to mold future leaders, Waseda continues to fulfill this mission, counting among its alumni seven prime ministers and countless other politicians, business leaders, journalists, diplomats, scholars, scientists, actors, writers, athletes and artists. The University is also number one in Japan in international activities, including the number of international students, with the broadest range of degree programs fully taught in English.















