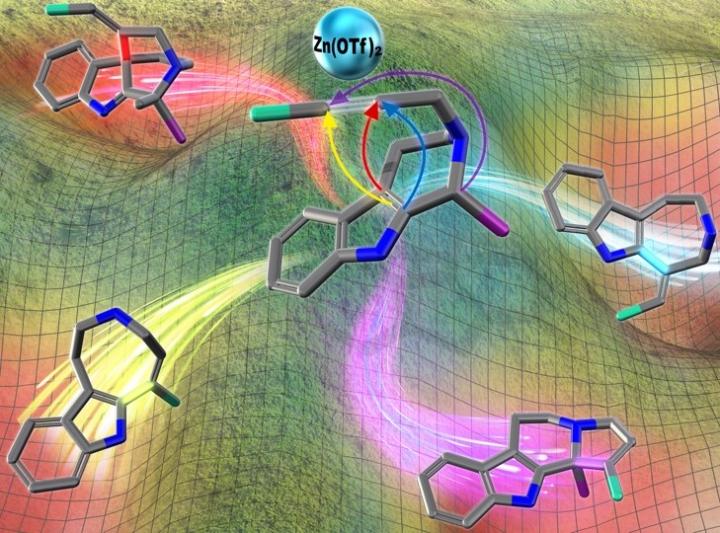
An artist's depiction of the programmable divergent synthesis process.
Credit: Hiroki Oguri, TUAT
This synthetic approach employing zing (II) reagent in place of Hg(II) or Gold-based reagents are also environmentally friendly as well as much cheaper than those that were used to-date.
The research was published in Chemical Science on May 1st, 2019.
Plants and fungi that contain indole alkaloids have a long history of use in traditional medicine. In efforts to synthesize the complex alkaloids, chemists usually develop a customized synthetic strategy for efficient construction of the single targeted scaffold.
In contrast, this synthetic approach allowed concise and divergent synthesis of skeletally diverse alkaloidal scaffolds employing a common multipotent intermediate upon activation of alkyne moiety with zinc (II) reagent.
“We have successfully achieved programmable synthesis of the four distinct alkaloidal skeletons by implementing divergent annulations. The four kinds of annulation modes were demonstrated to be controlled in a programmable manner by appropriate choices of the substituents in the vicinity of the reaction centers, optimization of solvents and reaction conditions.” says Hiroki Oguri, PhD, corresponding author and Professor at the Department of Applied Chemistry, Graduate School of Engineering, TUAT, Japan.
By integrating computational and experimental investigation methods, the researchers were able to not only gain insight into the reaction mechanism proposing unique transition states, but also to showcase a useful synthetic strategy for the concise and divergent access to the medicinally-relevant alkaloidal structures.
“These experimental findings underscore that Zn(OTf)2-mediated activation of alkynes provide relatively unexplored but versatile synthetic methodologies for the direct and flexible synthesis of alkaloidal scaffolds reminiscent of natural products,” Oguri adds.
The researchers plan to implement their methods to create compounds other than just indole alkaloids, and they envision that their method could offer both rational and unexpected guidelines for designing other similar reactions.
Integration of synthetic strategies for generating structural variations with a systematic computational approach for identifying unforeseen reaction pathways could provide a new route for advancing the combinatorial chemical synthesis of functional molecules. They also expect that this method will generate lead candidates for the development of next-generation pharmaceuticals and pesticides.
###
This research was supported by the Japan Science and Technology Agency (JPMJPR13K3), the Japan Society for the Promotion of Science (KAKENHI Grant No. 15H03117, Grant No. JPMJCR14L5), the Asahi Grass Foundation, and Astellas Foundation for Research on Metabolic Disorders.
For more information about the Oguri laboratory, please visit http://web.
About Tokyo University of Agriculture and Technology
Tokyo University of Agriculture and Technology (TUAT) is a distinguished university in Japan dedicated to science and technology. TUAT focuses on agriculture and engineering that form the foundation of industry, and promotes education and research fields that incorporate them. Boasting a history of over 140 years since our founding in 1874, TUAT continues to boldly take on new challenges and steadily promote fields. With high ethics, TUAT fulfills social responsibility in the capacity of transmitting science and technology information towards the construction of a sustainable society where both human beings and nature can thrive in a symbiotic relationship. For more information, please visit http://www.
Original publication:
Sadaiwa Yorimoto, Akira Tsubouchi, Haruki Mizoguchi, Hideaki Oikawa, Yoshiaki Tsunekawa, Tomoya Ichino, Satoshi Maeda and Hiroki Oguri*
“Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds”
Chemical Science 2019, 10, 5686-5698.
DOI: 10.1039/c9sc01507h
Contact:
Hiroki Oguri, Ph.D., Professor,
Department of Applied Chemistry,
Graduate School of Engineering, TUAT, Japan.
h_oguri@cc.tuat.ac.jp
Satoshi Maeda, Ph.D., Professor,
Department of Chemistry, Faculty of Science,
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Japan.
smaeda@eis.hokudai.ac.jp