
Shedding light on the dark proteome with IMB’s newest Adjunct Director
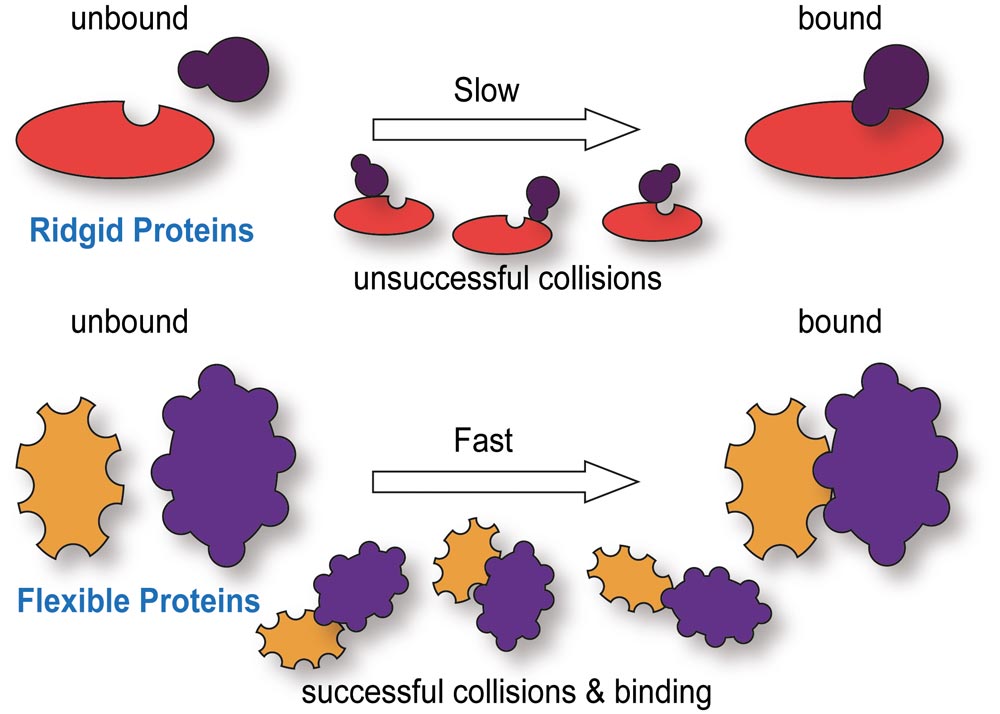
Comparison of ridgid and flexible proteins in their binding kinetics. (Top) Proteins with a ridged structure require interactions with binding partners to occur at highly specific sites. Random collisions make such interactions less common and reduce the speed for protein binding. (Bottom) Proteins which, on the other hand, are flexible in their native state can have many more binding sites and these sites are easier to access. This allows them to interact with more binding partners and they do so faster improving, for example, transport across the nuclear envelope.
Source: IMB
One of the first analogies that people learn when studying biology is that the specific interaction of proteins is like a lock and key. The 3D shape of the proteins determines their ability to interact and ensures that only desired interactions occur. But now imagine that your key is flexible and fluid.
Not only is deciphering the key’s shape impossible but it is hard to picture how such a key could ever be specific enough to be useful. The proteins in our cells are, in fact, often like this. It is estimated that up to 50% of the human proteome is comprised of proteins whose structures are fluid and unfolded in their native state. These proteins, known as intrinsically disordered proteins (IDPs), make up the “dark proteome”, as their level of molecular disorder has meant their structure cannot be elucidated with conventional techniques.
In the absence of a 3D structure, understanding the precise mechanism and function of a protein is simply much more difficult. Understanding these proteins is essential as, despite their flexible nature, their interactions can be very specific and crucial in vital cellular processes like nucleocytoplasmic transport, gene regulation and host pathogen interactions.
It is here, in the dark waters of the cell’s interior that Edward Lemke is shining a light, laser light to be specific. Edward has fused his expertise in both chemistry and biophysics to probe the structure and function of these IDPs at the single molecule level. “We develop technologies that permit the manipulation of biomolecules and the custom design of new functionalities into biology using advanced chemical and synthetic biology tools,” he says.
“Combining these technologies with custom designed single molecule probes and super-resolution instrumentation, we have been illuminating unique properties of IDPs that, for example, permit them to specifically but also rapidly shuttle proteins across the nuclear envelope” (see figure).
Following his appointment as Professor at JGU and as Adjunct Director at IMB, Edward’s Lab on “Synthetic Biophysics of Protein Disorder” will be bringing their expertise to Mainz in January 2018. Edward, who received an ERC Consolidator Grant in 2015, will continue to work on optimising the fluorescent labelling techniques he uses; establishing high throughput and microfluidic platforms for single molecule and super-resolution imaging; measuring the interactions of IDPs in real time; and focusing on IDPs that function in nuclear transport.
Image: https://www.imb.de/fileadmin/imb/groups/Lemke/Lemke_proteins_for_press_release.j…
Comparison of ridgid and flexible proteins in their binding kinetics. (Top) Proteins with a ridged structure require interactions with binding partners to occur at highly specific sites. Random collisions make such interactions less common and reduce the speed for protein binding. (Bottom) Proteins which, on the other hand, are flexible in their native state can have many more binding sites and these sites are easier to access. This allows them to interact with more binding partners and they do so faster improving, for example, transport across the nuclear envelope.
Further details
Further information about Edward’s work can be found at https://www.imb.de/research/lemke/research/ or http://www.lemkelab.com
Press contact for further information
Dr Ralf Dahm, Director of Scientific Management
Institute of Molecular Biology (IMB), Ackermannweg 4, 55128 Mainz, Germany
Phone: +49 (0) 6131 39 21455, Fax: +49 (0) 6131 39 21421, Email: press@imb.de












