Tarantula toxins converted to painkillers
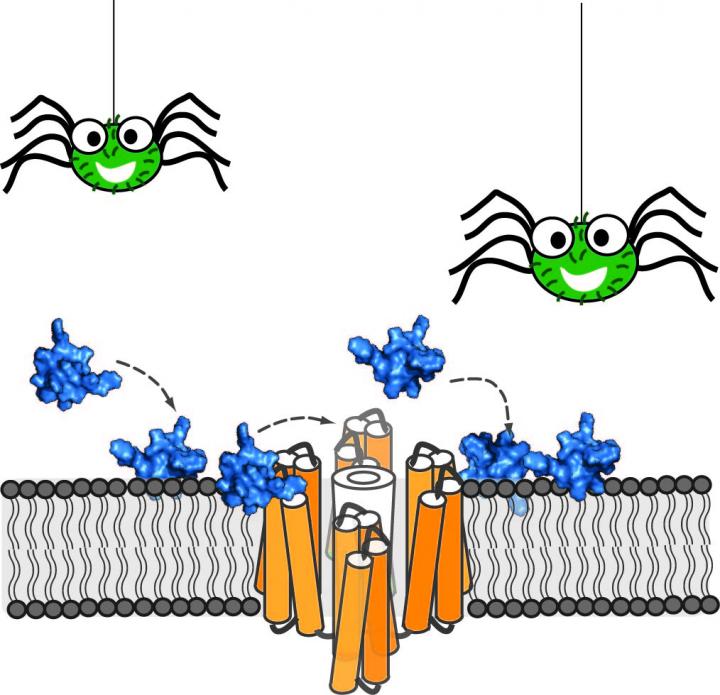
This is the peptide toxin (blue) isolated from the Peruvian green velvet tarantula binds to neuronal cell membranes and inhibits the pain receptor NaV 1.7 (orange) at the cell membrane surface. Credit: Henriques
When venom from animals such as spiders, snakes or cone snails is injected via a bite or harpoon, the cocktail of toxins delivered to its victim tends to cause serious reactions that, if untreated, can be lethal. But even venom has a therapeutic upside: Individual peptide toxins are being tapped to target receptors in the brain to potentially serve as painkillers.
Millions of people live with chronic and neuropathic pain, in large part because current treatments often provide limited pain relief, have a heavy profile of soporific side effects and can be extremely addictive. So researchers around the globe are chasing down potential new therapeutic agents and working to gain a better understanding of how molecules with painkiller activity function. This will lead to alternative painkillers–and possibly improve the quality of life for people who suffer from chronic pain.
At the Biophysical Society's 60th Annual Meeting, being held in Los Angeles, Calif., Feb. 27-March 2, 2016, a group of researchers from the University of Queensland in Brisbane, Australia, will describe their efforts with ProTx-II, a peptide toxin found within the venom of the Peruvian green velvet tarantula, Thrixopelma pruriens. Its high potency and selectivity to inhibit the pain sensation receptor make it an ideal candidate as a future painkiller.
“Our group is specifically interested in understanding the mode of action of this toxin to gain information that can guide us in the design and optimization of novel pain therapeutics,” said Sónia Troeira Henriques, senior research officer at the University of Queensland's Institute for Molecular Bioscience.
How does ProTx-II work? “It binds to the pain receptor located within the membrane of neuronal cells, but the precise peptide-receptor binding site and the importance of the cell membrane in the inhibitory activity of ProTx-II is unknown,” explained Henriques.
So the group zeroed in on its structure-activity relationship by “exploring the structure, the membrane-binding properties, and the inhibitory activity of ProTx-II and a series of analogues,” she added.
Nuclear magnetic resonance (NMR) spectroscopy enables 3-D characterization of the structure of this peptide, which allows the group to explore whether it's important for its ability to inhibit the pain receptor.
They also use surface plasmon resonance and fluorescence methodologies, as well as molecular simulations, to further characterize the interactions between the peptide and the neuronal cell membrane and to identify the molecular properties of the peptide involved in the interaction and inhibition with the pain receptor.
“Our results show that the cell membrane plays an important role in the ability of ProTx-II to inhibit the pain receptor. In particular, the neuronal cell membranes attract the peptide to the neurons, increase its concentration close to the pain receptors, and lock the peptide in the right orientation to maximize its interaction with the target,” said Henriques.
The group's work is the first to describe the importance of the membrane-binding properties of ProTx-II for its potency as an inhibitor of Nav 1.7, an important pain receptor. “Until now, studies characterizing the inhibitory activity of venom toxins have ignored the potential role of the cell membrane in their potency and activity,” she noted.
Beyond Nav 1.7, “other voltage-gated ion channels are located at the cell membrane and involved in a range of physiological processes such as muscle and nerve relaxation, regulation of blood pressure, and sensory transduction,” Henriques pointed out. “Their 'faulty' activity is, however, associated with several disorders, so other ion channels are actively being pursued as drug targets for the treatment of neuromuscular disease, neurological disorders, and inflammatory and neuropathic pain.”
Based on the group's findings, they're now designing new toxins with greater affinity for the cell membrane and fewer side effects.
“Our work creates an opportunity to explore the importance of the cell membrane in the activity of peptide toxins that target other voltage-gated ion channels involved in important disorders,” said Henriques.
Presentation #181, “Rational design and synthesis of a novel membrane binding NaV1.8 selective inhibitor with in vivo activity in pain models,” is authored by Christina I. Schroeder, Jennifer Deuis, Sonia Troeria Henriques, Zoltan Dekan, Marco Inserra, Mehdi Mobli and Irina Vetter. It will be at 4:00 p.m. PT on Sunday, Feb. 28, 2016 in Room 502B of the Los Angeles Convention Center. ABSTRACT: http://tinyurl.
###
MORE MEETING INFORMATION
ABOUT THE MEETING
Each year, the Biophysical Society Annual Meeting brings together more than 6,500 researchers working in the multidisciplinary fields representing biophysics. With more than 3,600 poster presentations, over 200 exhibits, and more than 20 symposia, the BPS Annual Meeting is the largest meeting of biophysicists in the world. Despite its size, the meeting retains its small-meeting flavor through its subgroup symposia, platform sessions, social activities and committee programs. The 60th Annual Meeting will be held at the Los Angeles Convention Center.
PRESS REGISTRATION
The Biophysical Society invites professional journalists, freelance science writers and public information officers to attend its Annual Meeting free of charge. For press registration, contact Ellen Weiss <EWeiss@biophysics.org> or the media line at the American Institute of Physics at <media@aip.org> or 301-209-3090.
NEWS RELEASES
Embargoed press releases describing in detail some of the breakthroughs to be discussed at the meeting are available on Eurekalert, Newswise and Alpha Galileo or by contacting the media line at the American Institute of Physics at media@aip.org or 301-209-3090.
QUICK LINKS
Main Meeting Page: http://tinyurl.
Symposia: http://tinyurl.
Itinerary planner: http://tinyurl.
ABOUT THE SOCIETY
The Biophysical Society, founded in 1958, is a professional, scientific Society established to encourage development and dissemination of knowledge in biophysics. The Society promotes growth in this expanding field through its annual meeting, monthly journal, and committee and outreach activities. Its 9,000 members are located throughout the U.S. and the world, where they teach and conduct research in colleges, universities, laboratories, government agencies, and industry. For more information on the Society, or the 2016 Annual Meeting, visit http://www.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

New organoid with all key pancreas cells
Researchers from the Organoid group (previously Clevers group) at the Hubrecht Institute have developed a new organoid that mimics the human fetal pancreas, offering a clearer view of its early development….

Unlocking the potential of nickel
New study reveals how to use single atoms to turn CO2 into valuable chemical resources. Nickel and nitrogen co-doped carbon (Ni-N-C) catalysts have shown exceptional performance in converting CO2 into…

‘Spooky action’ at a very short distance
Scientists map out quantum entanglement in protons. Particles streaming from collisions offer insight into dynamic interactions and collective behavior of quarks and gluons. Scientists at the U.S. Department of Energy’s…



