Two-dimensional melting of hard spheres experimentally unravelled after 60 years
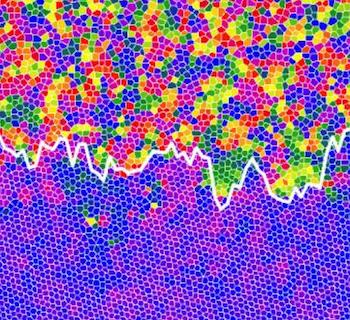
This is the interface between the liquid (top) and hexatic (bottom) states. Credit: Oxford University
After extensive research, scientists from the Department of Chemistry at the University of Oxford have found experimental evidence that sheds new light on the melting of two-dimensional substances. Findings from the study could be used to support technological improvements to thin film materials such as graphene.
Researchers from the group of Professor Roel Dullens at Oxford's Department of Chemistry have experimentally elucidated how melting of a two-dimensional solid of hard spheres occurs. With this work they resolve one of the most fundamentally important but still outstanding issues in condensed matter science. In addition, these results provide the cornerstone for the further understanding and development of two-dimensional materials.
Melting, the phase transition in which a substance turns from a solid to a liquid, is widely understood in basic terms. But despite being encountered regularly in everyday life, (whether in the workplace, home or natural world), scientists have long been trying to understand the melting process on a fundamental level.
The melting of a solid into a liquid is one of the most commonly experienced scientific phenomena. However, understanding this transformation is especially mysterious for solids in two-dimensions. Here, the celebrated Kosterlitz-Thouless-Halperin-Nelson-Young (KTHNY) theory proposes that an intermediate, partially disordered state, called the 'hexatic', exists between the solid and liquid.
Substantial effort has been made towards the understanding of these 'topological' transitions, for which Kosterlitz and Thouless were awarded the 2016 Nobel Prize in Physics [1,2]. Yet for the simplest interacting system of many particles, two-dimensional hard spheres, there has been an astonishing lack of consensus despite the first simulations being performed over 60 years ago.
Dr Alice Thorneywork and co-workers used optical microscopy to study monolayers of colloidal model hard spheres (see box 2) tilted by a small angle to introduce a gradient in the particle concentration [FIG 1]. For hard spheres, the behaviour is governed only by this concentration, which allowed them to identify and characterize the liquid, hexatic, and solid states and the nature of the transitions between them in a single experiment. The results show that the melting occurs via a continuous solid-hexatic transition followed by a first order hexatic-liquid transition [FIG 2].
###
[1] http://www.
Image info:
BOX 1: Two-dimensional hard spheres (similar make-up to a snooker ball triangle)
Hard spheres are simply solid balls that cannot overlap. When these spheres are confined to monolayer, just like the balls on a snooker table, this corresponds to a system of two-dimensional hard spheres. A collection of hard spheres is the simplest possible system that exhibits melting from a solid into a liquid.
BOX 2: Colloidal particles:
Colloidal particles have a typical size between a nanometre (a millionth of millimetre) and a micrometre (a thousandth of a millimetre). Spherical colloidal particles suspended in a liquid such as water are the best experimental realisation of micrometre-sized hard spheres (the scale bar in the image below corresponds to 1 micro-metre).
Fig 1:A microscopy image of the two-dimensional colloidal hard sphere system titled by a small angle ?.
Fig 2:The interface between the liquid (top) and hexatic (bottom) states.
The full study citation is as follows:
Two-Dimensional Melting of Colloidal Hard Spheres
Alice L. Thorneywork, Joshua L. Abbott, Dirk G.?A.?L. Aarts, and Roel P.?A. Dullens
Phys. Rev. Lett. 118, 158001 – Published 10 April 2017
For further information please contact Lanisha Butterfield in the University of Oxford press office at Lanisha.butterfield@admin.ox.ac.uk or on+44 (0)1865 280531
The Mathematical, Physical and Life Sciences Division (MPLS) is one of four academic divisions at the University of Oxford, representing the non-medical sciences. Oxford is one of the world's leading universities for science, and MPLS is at the forefront of scientific research across a wide range of disciplines. Research in the mathematical, physical and life sciences at Oxford was rated the best in the UK in the 2014 Research Excellence Framework (REF) assessment. MPLS received £133m in research income in 2014/15.
Media Contact
More Information:
https://eurekalert.org/pub_releases/2017-04/uoo-tmo042117.phpAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

New organoid with all key pancreas cells
Researchers from the Organoid group (previously Clevers group) at the Hubrecht Institute have developed a new organoid that mimics the human fetal pancreas, offering a clearer view of its early development….

Unlocking the potential of nickel
New study reveals how to use single atoms to turn CO2 into valuable chemical resources. Nickel and nitrogen co-doped carbon (Ni-N-C) catalysts have shown exceptional performance in converting CO2 into…

‘Spooky action’ at a very short distance
Scientists map out quantum entanglement in protons. Particles streaming from collisions offer insight into dynamic interactions and collective behavior of quarks and gluons. Scientists at the U.S. Department of Energy’s…



