
Molecular glue degraders – a new weapon to target “undruggable” cancer drivers
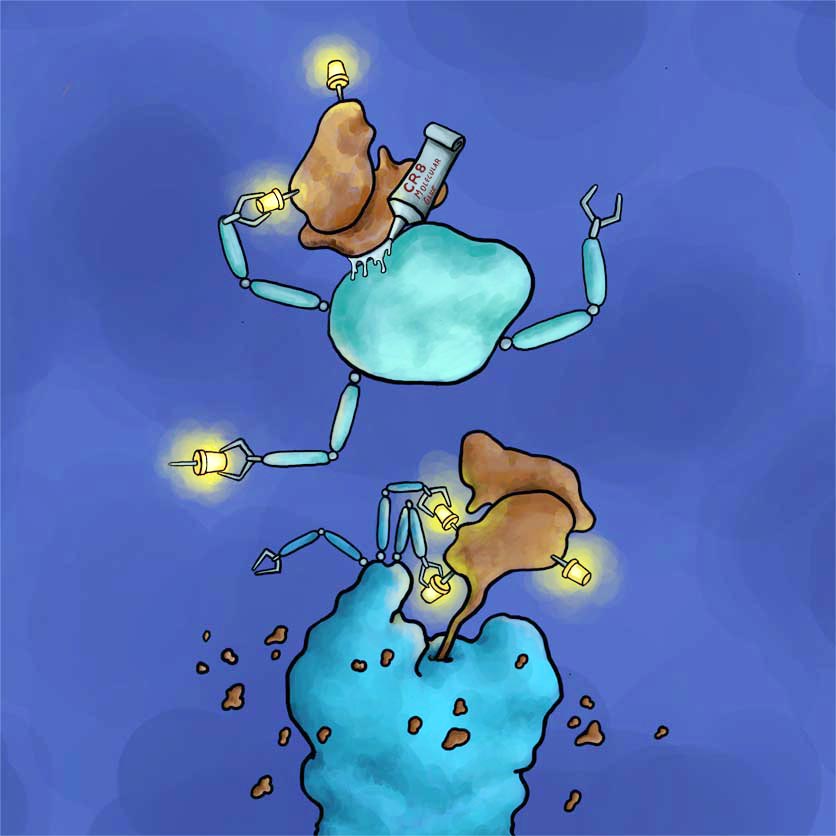
How a molecular molecular glue degrader works. The kinase inhibitor CR8 attaches the cancer-driving CDK12/cyclin K complex (brown) to an E3 ligase (green). This interaction causes cyclin K to be linked to ubiquitin molecules (yellow). Cyclin K molecules labeled in this way can be recognized and broken down by the cell's waste disposal system (blue).
Copyright: Jonas Koeppel
One strategy to fight cancer is to target its Achilles' heel – the addiction to growth-promoting proteins. However, as many of these so-called cancer drivers are notoriously difficult to block with a drug, this requires novel and unconventional approaches.
In cooperation with the German Cancer Research Center (DKFZ) and the National Center for Tumor Diseases (NCT) Heidelberg, researchers from Boston and Basel have elucidated the mechanism of action of one such unconventional drug.
This “molecular glue degrader” binds to a cyclin-dependent kinase and induces its interaction with the cell's waste disposal system, which in turn results in degradation of a cancer driver and subsequent growth inhibition.
The scientists' findings open up new paths in drug development.
he National Center for Tumor Diseases (NCT) Heidelberg is a joint institution of the German Cancer Research Center, Heidelberg University Hospital (UKHD) and German Cancer Aid.
Most targeted cancer drugs currently used in the clinic are inhibitors, small molecules that work by binding to and blocking the activity of enzymes. However, only a fraction of proteins critical for cancer growth have a suitable pocket for inhibition.
A novel approach to target such “undruggable” cancer drivers is to hijack the molecular machinery designed to remove aberrant proteins from the cell by degradation.
A vital component of the cell’s waste disposal system are E3 ligases, enzymes that regulate the abundance, and hence the activity of many proteins. In recent years, drugs were described that hijack E3 ligases to selectively destroy and remove disease-causing proteins.
The best-known example is thalidomide, which acts as a “molecular glue” that induces proximity between a transcription factor called Ikaros and an E3 ligase substrate receptor. By “gluing” these two proteins together, Ikaros is labeled with ubiquitin and thereby targeted for degradation.
A thalidomide analog called lenalidomide has been approved in Germany for the treatment of certain blood cancers, namely multiple myeloma and myelodysplastic syndrome.
“To identify new molecular glues, we investigated thousands of drugs for their ability to inhibit cancer cells in relation to the expression levels of E3 ligases”, reports Mikołaj Słabicki, postdoctoral researcher at the Dana-Farber Cancer Institute (DFCI) in Boston and in the Division of Translational Medical Oncology at DKFZ and NCT Heidelberg and co-first author of the study. “We discovered that the kinase inhibitor CR8 inhibits the growth of cancer cells by recruiting an E3 ligase to the growth-promoting CDK12/cyclin K complex, which results in rapid degradation of cyclin K”.
Upon closer characterization of CR8, the researchers found a structural similarity to a known kinase inhibitor called roscovitine or seliciclib. “We have shown that it is possible to take a conventional kinase inhibitor and, by attaching a particular chemical group, transform it into a molecular glue degrader,” said co-senior author Benjamin Ebert, Chair of the Department of Medical Oncology at DFCI and recipient of the 2019 Meyenburg Cancer Research Award.
The molecular details of the CR8-induced molecular glue complex were revealed by researchers from the Friedrich Miescher Institute for Biomedical Research (FMI) in Basel, including co-senior author Nicolas Thomä and co-first authors Zuzanna Kozicka and Georg Petzold.
The teams on both sides of the Atlantic concluded that a small chemical moiety is required for CR8 to act as a molecular glue degrader. “This could be a more generalizable strategy for drug design and result in new options for the treatment of cancer”, says Kozicka.
The quest for novel molecular glue degraders was a joint effort by scientists from DFCI, the Broad Institute of Harvard and Massachusetts Institute of Technology in Cambridge, and FMI in collaboration with investigators at DKFZ and NCT Heidelberg, including two Major Cancer Biology students, Manisha Manojkumar and Jonas Koeppel.
“This study was true detective work, with every experiment uncovering additional pieces of the puzzle. It required a multi-disciplinary effort by team members from various fields, including cancer biology, bioinformatics, functional genomics, structural biology, and biochemistry, and was an incredibly exciting and rewarding experience”, says Słabicki.
Original publication:
M. Słabicki, Z. Kozicka, G. Petzold et al. (2020) The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature. Online June 3, 3020 https://doi.org/10.1038/s41586-020-2374-x
Image for the press release are available free of charge on the Internet at
https://www.nct-heidelberg.de/fileadmin/media/nct-heidelberg/news/Meldungen/Bild…
Legend: How a molecular molecular glue degrader works. The kinase inhibitor CR8 attaches the cancer-driving CDK12/cyclin K complex (brown) to an E3 ligase (green). This interaction causes cyclin K to be linked to ubiquitin molecules (yellow). Cyclin K molecules labeled in this way can be recognized and broken down by the cell's waste disposal system (blue).
Terms of use for image material for press releases
The use is free of charge. The NCT Heidelberg permits one-time use in connection with reporting on the topic of the press release. Please indicate as picture credits: “Copyright: Jonas Koeppel”. The images may only be passed on to third parties after prior consultation with the NCT press office (Tel. 06221 56 5930, e-mail friederike.fellenberg@nct-heidelberg.de). Use for commercial purposes is prohibited.
Press contact:
Dr. Friederike Fellenberg
National Center for Tumor Diseases Heidelberg (NCT)
Press and Public Relations
Im Neuenheimer Feld 460
69120 Heidelberg
Tel.: +49 6221 56-5930
Fax: +49 6221 56-5350
Email: friederike.fellenberg@nct-heidelberg.de
Dr. Sibylle Kohlstädt
German Cancer Research Center (DKFZ)
Communications and Marketing
Im Neuenheimer Feld 280
69120 Heidelberg
Tel.: +49 6221 42-2843
Fax: +49 6221 42-2968
Email: s.kohlstaedt@dkfz.de
Doris Rübsam-Brodkorb
Heidelberg University Hospital and Medical Faculty of the University of Heidelberg
Press and Public Relations
Im Neuenheimer Feld 672
69120 Heidelberg
Tel.: +49 6221 56-5052
Fax: +49 6221 56-4544
Email: doris.ruebsam-brodkorb@med.uni-heidelberg.de
www.klinikum.uni-heidelberg.de
National Center for Tumor Diseases Heidelberg (NCT)
The National Center for Tumor Diseases (NCT) Heidelberg is a joint institution of the German Cancer Research Center, Heidelberg University Hospital (UKHD) and German Cancer Aid. The NCT's goal is to link promising approaches from cancer research with patient care from diagnosis to treatment, aftercare and prevention. This is true for diagnosis and treatment, follow-up care or prevention. The interdisciplinary tumor outpatient clinic is the central element of the NCT. Here, the patients benefit from an individual treatment plan prepared in interdisciplinary expert rounds, so-called tumor boards. Participation in clinical studies provides access to innovative therapies. The NCT thereby acts as a pioneering platform that translates novel research results from the laboratory into clinical practice. The NCT cooperates with self-help groups and supports them in their work. Since 2015, the NCT Heidelberg has maintained a partner site in Dresden. The Hopp Children's Cancer Center (KiTZ) was established in Heidelberg in 2017. The pediatric oncologists at KiTZ work together in parallel structures with the NCT Heidelberg.
German Cancer Research Center (DKFZ)
The German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) with its more than 3,000 employees is the largest biomedical research institution in Germany. More than 1,300 scientists at the DKFZ investigate how cancer develops, identify cancer risk factors and search for new strategies to prevent people from developing cancer. They are developing new methods to diagnose tumors more precisely and treat cancer patients more successfully. The DKFZ's Cancer Information Service (KID) provides patients, interested citizens and experts with individual answers to all questions on cancer.
Jointly with partners from the university hospitals, the DKFZ operates the National Center for Tumor Diseases (NCT) in Heidelberg and Dresden, and the Hopp Children's Tumour Center KiTZ in Heidelberg. In the German Consortium for Translational Cancer Research (DKTK), one of the six German Centers for Health Research, the DKFZ maintains translational centers at seven university partner locations. NCT and DKTK sites combine excellent university medicine with the high-profile research of the DKFZ. They contribute to the endeavor of transferring promising approaches from cancer research to the clinic and thus improving the chances of cancer patients.
The DKFZ is 90 percent financed by the Federal Ministry of Education and Research and 10 percent by the state of Baden-Württemberg. The DKFZ is a member of the Helmholtz Association of German Research Centers.
Heidelberg University Hospital (UKHD)
Heidelberg University Hospital (UKHD) is one of the most important medical centers in Germany; Heidelberg University's Medical Faculty is one of Europe's most prestigious biomedical research facilities. Their shared objective is the development of innovative diagnostics and treatments and their prompt implementation for the benefit of the patient. The hospital and faculty employ approximately 13,000 individuals and are involved in training and qualification. Every year approximately 65,000 patients are treated as inpatients and 56,000 as day patients in more than 50 specialized clinical departments with around 2,000 beds, with more than 1 million patients being treated as outpatients. Together with the German Cancer Research Center and German Cancer Aid, the Heidelberg University Hospital established The National Center for Tumor Diseases (NCT) Heidelberg as the leading oncology center of excellence in Germany. The Heidelberg Curriculum Medicinale (HeiCuMed) is at the forefront of medical training in Germany. At present 3,700 aspiring physicians and doctors are studying in Heidelberg.
Mikołaj Słabicki, Benjamin Ebert
M. Słabicki, Z. Kozicka, G. Petzold et al. (2020) The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature. Online June 3, 3020 https://doi.org/10.1038/s41586-020-2374-x












