
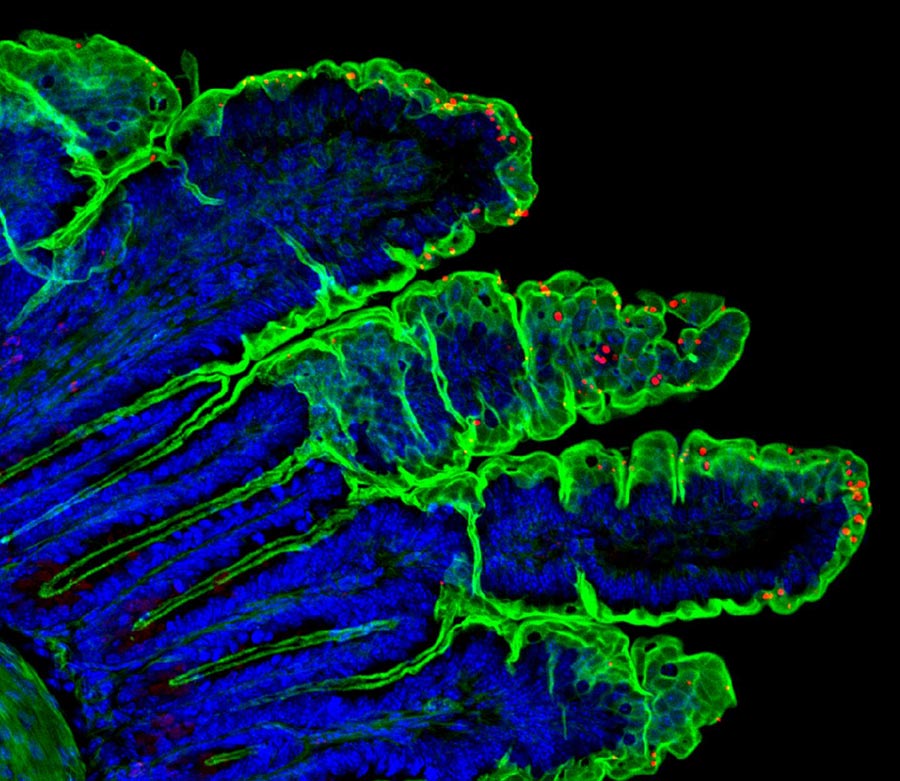
The parasite Cryptosporidium, transmitted through water sources, is one of the most common causes of diarrheal disease in the world. New inroads into modeling the disease--and preventing it with a vaccine--lay the groundwork for tackling the infection in humans. Here, A section of intestine from an infected mouse shows Cryptosporidium tyzzeri parasites in red. The University of Pennsylvania-led team is the first to sequence, study, and manipulate a naturally occurring mouse Cryptosporidium.
Credit: Muthgapatti Kandasamy, Adam Sateriale, and Boris Striepen
Usage Restrictions: In context of reporting
The intestinal parasite Cryptosporidium, which causes a diarrheal disease, is very good at infecting humans. It's the leading cause of waterborne disease from recreational waters in the United States.
Globally, it's a serious illness that can stunt the growth of, or even kill, infants and young children. And people with compromised immune systems, such as those with HIV/AIDS, are also highly susceptible. There is no vaccine and no effective treatment.
Surprisingly, the parasite strains that infect humans don't do such a good job at infecting mice. To study the disease, researchers have had to rely on mice with defective immune systems, a model that made it difficult to understand how to elicit an immune response that could protect children.
But that is set to change. Using a naturally occurring species of mouse Cryptosporidium, a team led by researchers from Penn's School of Veterinary Medicine has developed a model of infection that affects immunologically normal mice. They show that mice develop immunity to the parasite after infection, and that a live attenuated vaccine offers the animals protection against it. Their findings appear in the journal Cell Host & Microbe.
“We now have a fantastic mouse model that mirrors the human disease,” says Boris Striepen, a biologist at Penn Vet and senior author on the study. “It's a powerful lab model, where we can introduce changes at will and test the importance of different components of the immune response to infection, which is just what we need to develop an effective vaccine.”
Mice that received the experimental vaccine, which used a weakened version of the parasite, were as protected from infection as those that had already weathered an initial infection, the researchers found. “We were able to show that the mice were protected –not by sterile immunity–but by very robust protection from disease, which is exactly what is observed in children,” says Adam Sateriale, first author on the report and a postdoctoral researcher in Striepen's lab.
Striepen has focused on advancing science on Cryptosporidium for the last several years. One major advance came in 2015, when his lab found success in using the CRISPR-Cas9 technology to genetically modify the organism.
In the new work, Striepen, Sateriale, and colleagues aimed to develop a method to more easily study the parasite in mice, which are resistant to the two species responsible for most human infections. Taking a different tack, they searched for Cryptosporidium DNA in mice feces from farms and found one species, C. tyzzeri, in 30 percent of the samples.
“One of the first things we did was sequence and annotate the genome,” says Sateriale, finding it to be an extremely close relative of the species that affect humans. “Once we know the genome, we can not only see how it varies compared to those species, but we can also begin to use our genetic tools to manipulate it.”
Among the manipulations the researchers made using CRISPR were introducing genes that make the parasite glow using a gene borrowed from the firefly, allowing them to precisely, but non-invasively, track the infection.
Unlike the more artificial models of infection that used immunocompromised mice, the Penn-led team showed that C. tyzzeri could infect healthy mice, causing an infection that replicated many features of human disease.
“Some of the main immunological components that have been shown to be important in people were also true of this mouse model,” Striepen notes.
Specifically, they found that T cells and the protein interferon-gamma, a key player in fighting off a variety of infections, were both critical in the body's response to the parasite. Mice lacking the gene for interferon-gamma and those that lacked T cells had more severe, longer-lasting infections than normal mice.
“Understanding these correlates of immunity–how the parasite triggers an immune response, and by what mechanism the immune system then attacks the parasite–are important aspects of vaccine development,” Striepen says.
Knowing that children who become infected with Cryptosporidium can develop resistance to subsequent infections, the researchers wanted to see if the same held true in the mice. After confirming that this was the case, their final effort was to attempt to vaccinate the mice. They exposed C. tyzzeri spores to radiation to weaken them. Mice that received the vaccination with the live attenuated C. tyzzeri were protected from infection, though mice lacking either interferon-gamma or T cells were not protected, again underscoring the importance of these factors in developing anti-Cryptosporidium immunity.
Encouraged by their findings, the researchers are continuing to probe the pathways involved in conferring immune protection against Cryptosporidium infection, and are sharing their model with colleagues to aggressively pursue a vaccine or other treatments for the disease.
“We feel fortunate to be at the vet school and at Penn in general as we work on these questions,” says Striepen. “Here we can build larger teams of parasite biologists, and experts in the study of immune responses like our colleague Christopher Hunter, so we're building up an interdisciplinary effort that can overcome the challenges of working on these complex investigations. And hopefully this will lead to advances that protect children.”
###
Striepen and Sateriale collaborated on the work with Penn Vet's Julie B. Engiles, Jodi A. Gullicksrud, Emily M. Kugler, and Christopher A. Hunter; the University of Sydney's Jan Šlapeta; and the University of Georgia's Rodrigo Baptista, Gillian T. Herbert, Carrie F. Brooks, and Jessica C. Kissinger.
Boris Striepen is a professor in the Department of Pathobiology in the University of Pennsylvania School of Veterinary Medicine.
Adam Sateriale is a postdoctoral researcher in the Striepen laboratory in Penn's School of Veterinary Medicine.
The study was supported in part by the National Institutes of Health (grants AI112427, AI124518, AI055400, and AI137442) and the Bill and Melinda Gates Foundation (grants OPP1161001 and OPP1151701).












