
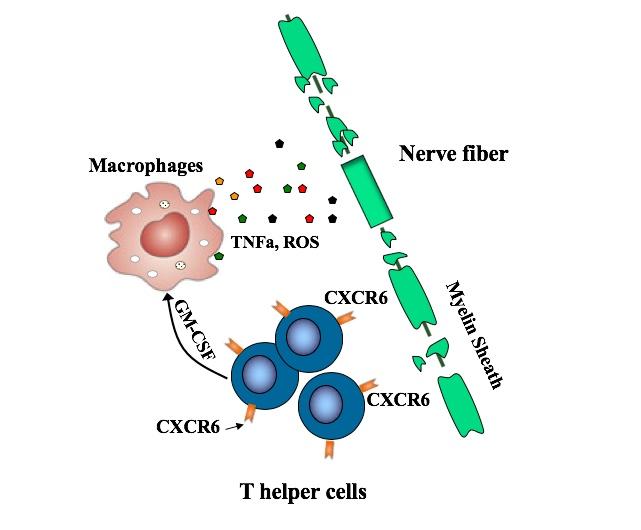
T helper cells bearing the CXCR6 surface marker drive multiple sclerosis by producing a host of proteins that damage nerve fibers, by attacking their protective myelin sheath. The cells are shown here releasing GM-CSF, which stimulates other immune cells, macrophages, to release damaging inflammatory compounds.
Credit: Lifei Hou/Boston Children's Hospital
Multiple sclerosis, an autoimmune disorder, is known to be driven by “helper” T cells, white blood cells that mount an inflammatory attack on the brain and spinal cord. A new study from Boston Children's Hospital pinpoints the specific subgroup of helper T cells that cause MS, as well as a protein on their surface, called CXCR6, that marks them. An antibody targeting CXCR6 both prevented and reversed MS in a mouse model, the researchers report this week in PNAS.
If the findings bear out in human studies, targeting these rogue T cells could ameliorate MS, the researchers believe. The findings could also apply to other forms of autoimmune encephalomyelitis (inflammation of the brain and spinal cord), as well as inflammatory arthritis, says Eileen Remold-O'Donnell, PhD, of the Program in Cellular and Molecular Medicine at Boston Children's Hospital, senior author on the paper.
Remold-O'Donnell and Lifei Hou, PhD, her former postdoctoral fellow and first author on the paper, have filed a patent covering the work and have formed a company, Edelweiss Immune, Inc., in which they have equity ownership together with Boston Children's Hospital.
“We've demonstrated in mice you can target these cells and get rid of them,” says Remold-O'Donnell, a principal in the company together with Hou. “We don't know if this approach would be appropriate for all cases of MS, but it could be effective in the early inflammatory stages of the disease, and in targeting newly formed cells during disease exacerbations.”
Targeting MS-inducing cells
T helper cells in general have been known to drive MS, coordinating the attack on the protective myelin sheath that covers nerve fibers. But there are many different types of T helper cells. Recent studies have pointed to TH17 cells, but some TH17 cells appear not to be involved in MS.
The new study zeroed in on a subset of TH17-derived cells, all bearing the CXCR6 marker. These cells are fast-proliferating and very damaging, producing one set of proteins that directly damage cells and others, including GM-CSF, that stimulate an inflammatory attack by other immune cells known as macrophages.
The study showed that these cells also produce increased amounts of a protein called SerpinB1 (Sb1), and that this protein's presence is necessary for MS symptoms. When Sb1 was genetically deleted in T cells in the mouse MS model, fewer immune cells survived to infiltrate the spinal cord, and the disease was ameliorated as compared with control mice. The team then went on to show that these Sb1-containing cells could be readily identified with antibodies targeting the CXCR6 surface protein.
Human counterparts
To investigate whether CXCR6-positive cells are relevant in human disease, Remold-O'Donnell and Hou worked with physicians in Boston Children's departments of Immunology and Neurology, as well as rheumatologists at Brigham and Women's Hospital, to obtain samples of synovial fluid (from the cavities of joints) from patients with inflammatory autoimmune arthritis. They indeed found elevated levels of CXCR6+ cells in the inflamed joints. In contrast, circulating blood from the arthritis patients did not have elevated CXCR6+ cells. Nor did the blood of patients with MS or from healthy controls.
When the team used monoclonal antibodies to target CXCR6, the harmful cells largely disappeared, and mice, which were primed to get MS, did not develop the disease.
The researchers believe treatments to deplete CXCR6+ cells could mitigate MS and possibly other autoimmune disorders while largely leaving other T cell immune defenses intact. The new company, Edelweiss Immune, will be carrying the research forward.
“Many drugs have been developed to treat autoimmune diseases, such as glucocorticoids and cytotoxic reagents,” says Hou. “However, none selectively target pathogenic T cells, and long-term use of immunosuppressive agents results in broad immunosuppression and compromised immune defenses. Therapeutics with better selectivity, safety, and efficacy are needed.”
###
Coauthors on the study at Boston Children's were Koichi Yuki of the Department of Anesthesiology, Critical Care and Pain Medicine; Jessica Cooley of the Program in Cellular and Molecular Medicine; Lauren Henderson and Peter Nigrovic of the Rheumatology Program; and Mark Gorman of the Department of Neurology. See the paper for a full list of authors. The work was supported by the NIH (R21 AI117440, RO1 AR065538, KO8 AR073339, K08 AR072791, P30 AR070253) and the Swiss National Science Foundation (316030_150768, 310030_146130).
About Boston Children's Hospital
Boston Children's Hospital is ranked the #1 children's hospital in the nation by U.S. News & World Report and is the primary pediatric teaching affiliate of Harvard Medical School. Home to the world's largest research enterprise based at a pediatric medical center, its discoveries have benefited both children and adults since 1869. Today, 3,000 researchers and scientific staff, including 8 members of the National Academy of Sciences, 18 members of the National Academy of Medicine and 12 Howard Hughes Medical Investigators comprise Boston Children's research community. Founded as a 20-bed hospital for children, Boston Children's is now a 415-bed comprehensive center for pediatric and adolescent health care. For more, visit our Discoveries blog and follow us on social media @BostonChildrens, @BCH_Innovation, Facebook and YouTube.













After an MRI i was diagnosed of MULTIPLE SCLEROSIS. After years on medications, symptoms worsened with tremors on my right hand, numbness and tingling, muscle weakness and loss of speech. Fortunately last year, I learnt about Mayaka Natural Clinic (ww w. mayakanaturalclinic. c om) and their effective MS Formula treatment through an MS support group on facebook the Multiple Sclerosis treatment made a great difference, most of my symptoms including balance, weakness, falling alot and others gradually disappeared. I improved greatly over the 4 months treatment, its been a year since the treatment, i have no symptoms. I have a very good quality of life and a great family!