
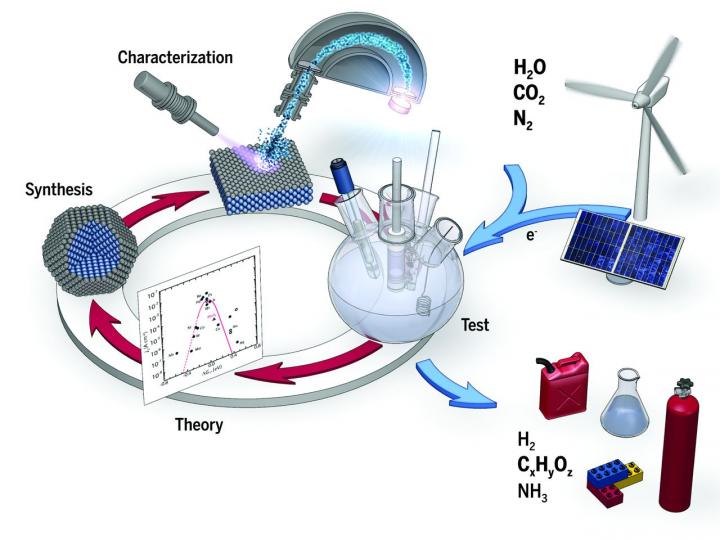
Schematic showing electrochemical conversion of water, carbon dioxide, and nitrogen into value-added products (e.g., hydrogen, hydrocarbons, oxygenates, and ammonia), using energy from renewable sources. The combination of theoretical and experimental studies working in concert provides us with insight into these electrochemical transformations and guides the development of the high-performance electrocatalysts needed to enable these technologies.
Credit: Jakob Kibsgaard, DTU Physics
Demand is not likely to fall, so we need to find a way to make the fuels and chemicals that are an integrated part of our everyday lives in a sustainable and fossil-free way. The transport sector accounts for approx. 19 per cent of global energy demand, while the production of chemicals accounts for around 8 per cent.
Electrocatalysis could be the way forward
One way to escape dependence on oil is through electrocatalysis, which can transform molecules in our atmosphere (such as water, CO2, and nitrogen) into more expensive and useful products, such as hydrogen or methanol (fuel), and chemicals–like ammonia (used in fertilizers).
In a new article just published in the Science journal, researchers from DTU and Stanford University in USA examine the current status of the field of electrocatalysis, and what it will take for the technology to be further developed.
“The big question is how to give renewable energy a greater role in our society. We have plenty of wind energy in Denmark, and the prices of solar energy have been falling over the last few years. In some areas it is becoming cheaper than generating electricity by conventional means,” says Assistant Professor Jakob Kibsgaard from DTU Physics, who co-authored the article.
“To get these sources to play a greater role, we will take electricity from renewable sources and basic molecules that exist in the atmosphere–such as water, CO2 or nitrogen. The electricity will drive an electrocatalytic process that converts the molecules into something useful. Water can be split into hydrogen and oxygen, and hydrogen can be used in hydrogen vehicles powered by fuel cells. A second reaction is to take CO2 and reduce it to methanol or ethanol, which can be incorporated directly into our fuel distribution as it now stands.”
As a third example, researchers are looking for a good catalyst that can make ammonia, used in artificial fertilizers. This is currently done using a chemical process that requires a very large plant. This means that while artificial fertilizer is relatively cheap in the western world, it is far more expensive in Africa because it has to be imported. If we could simplify the process and use electrocatalysis, we could have a more decentralized supply of artificial fertilizers–powered by a nearby solar plant using nitrogen taken from the air.
Lack of catalytic converters
The processes behind electrocatalysis can easily be powered by renewable energy sources, such as solar and wind power, and we therefore have the potential to make the world less dependent on fossil resources. But these processes are not yet how we want them to be. The catalysts currently available are not good enough. Effective catalysts are needed in order to drive the electrocatalytic processes, and these are either not good enough or not yet identified.
For example, nitrogen molecules (N2) are bound together very strongly, so it requires a good catalyst before the process can proceed. And this catalyst has not yet been found. Regarding the catalysis of water and CO2, we are still a long way from a big breakthrough.
The technology is not yet adequate for renewable energy to make a big splash–the authors of the article conclude, arguing that an important condition for success is a stronger connection between theory and experiments.
“In essence, when searching for a good catalyst I can simply start from one end of the periodic table with its 118 elements. These can then be mixed in thousands of ways. Analysts can help make the list much shorter, reducing it from hundreds of thousands of materials to perhaps 10. And that gives me a place to start,” says Jakob Kibsgaard.
“We can then begin to synthesize the materials, determine their characteristics, and finally see whether they work as we want. I can send this information back to the analysts, who can adjust their models. Through this iterative process, we will hopefully identify the materials that are both effective and stable. This is basically the method that has proven to work in the past.”
Splitting water shows potential
Jakob Kibsgaard notes that one of the best examples has been the development of a catalyst to extract hydrogen by splitting water. The best strategy in this field has been a combination of theoretical and experimental studies. This approach has led to the derivation of certain general principles that have improved our understanding of what materials can be used to drive the catalytic process.
Platinum is currently the best catalyst for the process. But it is scarce and very expensive (some claim that if all the mined platinum in the world was poured into an Olympic swimming pool, it would only reach ankle depth). A lot of work has therefore been done to find a replacement. And this has been partly successful. Almost ten years ago, a study by the same group at DTU Physics showed that molybdenum disulfide was a potential substitute for platinum. Molybdenum disulfide is a layered graphite-like material which has been used as a solid lubricant, among other applications. It had previously been seen as quite inert, but the study showed that the edges of the layers could serve as an active catalyst.
“This has launched a new field within catalysis. People all over the world are working to create various molybdenum disulfide nanostructures with as many edges as possible. These have become increasingly effective over the years, so we have now come very close to the same electrode activity as seen for platinum,” says Jakob Kibsgaard.
But this is only the hydrogen side of the catalytic process. Water also contains oxygen, and an optimal catalyst has not yet been found for this. Every time researchers optimize one parameter, another parameter deteriorates. This has been going on for over ten years. As long as the oxygen catalysis fails to show signs of becoming effective, the energy consumption of the whole process is too high–making hydrogen production less competitive. Analysts are again needed to look at which materials can be used and what shape the oxygen catalyst should have in order to crack the code.
The same issues essentially apply to CO2 catalysis into methanol etc. and nitrogen into ammonia.
Theory and experiments must be linked
“We work with analysts who present ideas for what could be interesting, and there are some exciting ideas. We have to think outside the box, as there are many factors that play a role when designing a catalyst. Once you have decided which elements to use, you can choose to arrange them as nanowires, three-dimensional porous structures or nanoparticles. You can then consider whether you want cubic, pyramid-shaped or round particles, and whether they should be mixed into alloys. So there are many parameters to adjust,” says Jakob Kibsgaard.
One of the main conclusions of the article is that there is a need to further strengthen the link between the theoretical and experimental level. This will require new computer models that can quickly calculate how different materials react in the catalytic process, and how they are affected by various circumstances.
“We are striving to understand the catalytic systems in depth, and how to create a good catalyst. We need to build a foundation for how we develop these catalysts. As electricity from renewable energy becomes cheaper and cheaper, it also increases the motivation to use it for much more than we do today. Not least because it would be good to reduce our carbon emissions,” he says.
“Electrocatalysis has the potential to be one of the ways we can use renewable energy to produce fuels for heavy transport and air travel, chemicals for fertilizers, and molecules to be used in the production of plastics. For example, if we find a catalyst that can make ethanol cheaply–and ideally from CO2–it would be a real game changer.”












