A new model of quantum dots: Rethinking the electronics
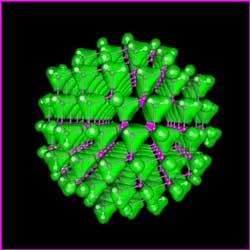
The total electron charge density (shown in green) of a quantum dot of gallium arsenide, containing just 465 atoms. (Image: Lin-Wang Wang)
Quantum dots, tiny crystals consisting of a few hundred to a few thousand atoms, sparkle with promise for uses ranging from tagging proteins in living cells to foiling counterfeiters to enabling quantum computers. The optics and electronics of these semiconductor nanocrystals are dramatically different from the same materials in bulk. But it turns out that one of the most important electronic properties of quantum dots has been misunderstood for over a decade.
Theorists at the Department of Energy’s Lawrence Berkeley National Laboratory have shown that a quantum dot’s dielectric function (a term indicating how charge responds to an electric field) does not depend on its band gap, as researchers long believed. On the contrary, the dielectric function of a quantum dot, measured on the microscopic scale, is virtually the same as that of the bulk material — except near the dot’s surface. “One of the interesting things about quantum dots is that their band gaps are much larger than the same material in bulk. At the same time their overall dielectric constants are much smaller,” says Lin-Wang Wang of Berkeley Lab’s Computational Research Division. “Therefore it was natural to assume that the size of the band gap in a quantum dot is what determines its overall dielectric constant.”
Recently French researchers led by Christophe Delerue of the Institut Supérieur d’Electronique du Nord raised doubts about this assumed relationship, however, basing their argument on approximate calculations. To test the questions posed by the French group, Wang and postdoctoral fellow Xavier Cartoixà performed, for the first time, ab initio (“from first principles”) microscopic studies of the dielectric function in quantum dots. To do so they used PEtot, a quantum-mechanical electronic-structure program developed by Wang, on the Seaborg supercomputer at the Department of Energy’s National Energy Research Scientific Computing Center (NERSC), based at Berkeley Lab.
Wang and Cartoixá’s results, published in the June 17, 2005 issue of Physical Review Letters, led them to devise a simple mathematical model, the first that nanoscience researchers can use for quick, consistent calculations of the dielectric function in nanocrystals.
Tunable band gaps and a rainbow of colors
“One useful feature of quantum dots is that the colors of light they absorb and emit can be tuned simply by varying their size,” says Wang. “This is because dots of the same material but different sizes have different band gaps, which absorb and emit different frequencies.”
The band gap of a semiconductor like silicon or gallium arsenide is the energy required to lift an electron from its valence band, filled with electrons, to its conduction band, which is empty. For example, an incoming photon whose energy matches or exceeds the band gap can boost an electron into the conduction band, leaving behind a “hole” of opposite charge. This is the principle that underlies photovoltaic cells, which generate electrical current when stimulated by light.
Conversely, when an electron falls from the conduction band back down to the valence band, eliminating a hole, the lost energy is emitted as light whose color corresponds to the band gap — this is the principle behind light-emitting diodes, LEDs.
Each semiconductor has a characteristic band gap, but when the diameter of a piece of the material is shorter than the quantum-mechanical wave function of its electrons, the “squeezed” electron wave function makes the band gap wider. For an electron to jump from the valence band to the conduction band now requires more energy. “In a classical picture this would be like the electron, which is free to meander through the bulk material, suddenly being forced to speed up in a confined space,” Lin-Wang Wang says — analogous to a circus motorcycle rider moving faster inside a steel cage.
The smaller the quantum dot, the wider the band gap. The band gap of gallium arsenide in bulk, for example, is 1.52 electron volts (eV), while a quantum dot consisting of 933 atoms of gallium and arsenic has a band gap of 2.8 eV, and a dot half as big, with 465 atoms, has a band gap of 3.2 eV — about twice that of the bulk material. Changing the band gap, and thus the color of light a quantum dot absorbs or emits, requires only adding or subtracting atoms from the quantum dot.
Enter the dielectric constant
The electron-hole pair formed when an incoming photon boosts an electron out of the valence band into the conduction band is called an exciton. An exciton’s energy (which corresponds to the color of the quantum dot) is not identical with the band gap; instead it depends on a number of other factors.
Most important is the dielectric function inside the quantum dot, which mediates how strongly the exciton’s negatively charged electron and positively charged hole attract each other. Calculating the dielectric function is thus essential to understanding how excitons behave in a quantum dot (including its exact color) and how its electronic states can be manipulated — for example by adding dopant atoms that seed the semiconductor with extra electrons or holes.
In 1994 Wang, then at DOE’s National Renewable Energy Laboratory, and his colleague Alex Zunger found a consistent relationship between a quantum dot’s band gap and its overall dielectric constant, a relationship suggestive of the observed scaling between a dot’s size and its band gap. A quantum dot’s electric constant is the average of the dielectric function inside the dot. Advances in computing now make it possible to calculate the dielectric function on the microscopic scale — virtually atom by atom.
In the recent study, Wang and Cartoixà calculated what would happen if a single-electron “perturbation” — caused by a dopant atom, for example — were introduced into the center of a 933-atom quantum dot of gallium arsenide. To replicate a realistic quantum dot, they “passivated” the atoms on its surface with fractionally charged hydrogen-like atoms, mimicking reactions between the dot and its surroundings.
Using the Seaborg supercomputer at NERSC, the researchers were able to determine the electron charge density of the perturbation throughout the dot, using an ab initio calculation technique called local density approximation. In the presence of a weak electric field the results were virtually identical to similar measurements of the bulk material — at least until the responses were measured near the surface of the dot.
They repeated the calculations for a 465-atom gallium-arsenide quantum dot, and also for a 465-atom quantum dot made of silicon. In the smaller dots, measurements near the center of the dot were still similar to the bulk measurements — but varied significantly where the perturbation vanishes, near the surface.
A simple model
Measured microscopically, the dielectric function inside a quantum dot is the same as it is in the bulk material; measurements near a perturbation in the center of the dot show no significant difference, but in a small dot the differences are large near the boundary. Averaging makes it appear that the dielectric constant mimics size-dependent changes in the band gaps. But in fact there is no direct relationship.
“Using many hours of supercomputer time, we calculated all the electronic states in these quantum dots when they were perturbed by a single electron in the middle,” says Wang. “We found they were the same as in the bulk.” The electronic response of a quantum dot thus depends on where it is measured, and on the dot’s size.
“If the response of the dot had been different from the bulk, it would have been hard to model,” Wang says. “Instead we were able to devise a simple model for calculating the dielectric function on the microscopic scale that gives virtually the same results as ab initio calculations with a supercomputer. This should be very useful in future calculations.” “Microscopic response effects in semiconductor quantum dots,” by Xavier Cartoixà and Lin-Wang Wang, appears in the June 17, 2005, issue of Physical Review Letters (volume 94, number 23, article 236804) and is available online as of June 15 at http://prl.aps.org/.
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California.
Media Contact
More Information:
http://www.lbl.govAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Magnetic tornado is stirring up the haze at Jupiter’s poles
Unusual magnetically driven vortices may be generating Earth-size concentrations of hydrocarbon haze. While Jupiter’s Great Red Spot has been a constant feature of the planet for centuries, University of California,…

Cause of common cancer immunotherapy side effect s
New insights into how checkpoint inhibitors affect the immune system could improve cancer treatment. A multinational collaboration co-led by the Garvan Institute of Medical Research has uncovered a potential explanation…

New tool makes quick health, environmental monitoring possible
University of Wisconsin–Madison biochemists have developed a new, efficient method that may give first responders, environmental monitoring groups, or even you, the ability to quickly detect harmful and health-relevant substances…



