Purdue creates self-generating nanotubes with ’dial-up’ properties
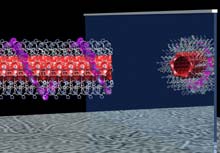
A self-assembled rosette nanotube and its mirror image prepared in the Fenniri laboratory. These materials are now made with predefined chiroptical, physical and chemical properties. The Fenniri group’s nanotubes promote their own formation and offer numerous potential applications. <br>(Purdue University Department of Chemistry) <br>
Nanotubes, stringy supermolecules already used to create fuel cell batteries and tiny computer circuits, could find myriad new applications ranging from disease treatment to plastics manufacturing to information storage, reports a Purdue University research team.
Scientists led by Purdue chemist Hicham Fenniri have learned to create multiple species of nanotubes that possess unprecedented physical and chemical properties, each of which could lead to a different industrial application. Also unprecedented is the compete control they have over the nanotubes’ formation, which allows the team to virtually “dial up” the properties they wish their nanotubes to possess. The findings could greatly expand the materials available for use on the nanoscale.
“Instead of being limited to building blocks of one size, shape and color, it’s as though we now have a brickyard with many different varieties,” Fenniri said. “This research could give a nanotechnologist a lot more materials for construction.”
The research will appear Saturday (8/24) on the Journal of the American Chemical Society’s Web site. A print publication date has not yet been determined.
Since their discovery in 1991, nanotubes have become one of the most promising building blocks for nanotechnology. Last year, Japan’s NEC Corp. developed a nanotube-based fuel cell battery that could power a notebook computer for days rather than hours. At about the same time, IBM researchers found a way to create logic circuits from individual carbon nanotubes, which could make them an alternative to silicon in future computers. After several years of pursuing their own research, Fenniri’s group has discovered a new class of nanotubes that could dramatically expand their uses in industry.
Rather than work with carbon or metals, as other groups have done, the Fenniri team has formed nanotubes out of synthetic organic molecules. While other materials have distinct advantages, they are not as easily managed as the materials the Fenniri team is working with.
“By using synthetic chemistry, we have gained complete control over the formation of our nanotubes,” Fenniri said. “More control in the lab should give more options to industry.”
One way the new nanotubes can be customized is by using them as scaffolding for other materials. Fenniri’s nanotube looks like a spiral-shaped stack of rings; each ring is made of six molecules shaped roughly like pie wedges. On the outside of the spiral, the team has learned to attach other molecules, which hang off the tubes like charms on a charm bracelet. The attached molecules then lend their properties to the outside of the nanotube.
For example, if the component molecules of nylon are attached, the nanotubes can then be turned into very long and flexible fibers that are, nonetheless, very strong.
“They could be made into an improved version of nylon,” Fenniri said. “And nylon has a lot more uses than making your socks stretch. We could use these fibers to reinforce everything from boat hulls and aircraft to body armor and parachutes.”
Another secret to creating custom-made tubes lies in manipulating a property called chirality, which has to do with the direction the spiral-shaped tubes twist. Nature only twists molecules in one direction – this is why DNA molecules always twist to the right, and are described as having right-handed chirality. But Fenniri’s team can make tubes that twist in either direction, creating left-handed nanotubes with abilities that their right-handed cousins often do not have.
“We can create two nanotubes that are made of the same materials, but that behave differently,” Fenniri said. “Just like a flipped-over puzzle piece doesn’t fit in its hole, a left-handed nanotube can react with different substances than its corresponding right-handed tube.”
While experimenting with controlling their nanotubes’ properties, the Fenniri team discovered some unexpected behaviors their nanotubes exhibit.
“We have found that the nanotubes promote their own formation,” Fenniri said. “Such behavior is very reminiscent of living systems, in that they replicate and adapt to their environment.”
Realizing that their homegrown nanotubes catalyze their own formation opened a whole new field of research for the team. They found that by placing the raw materials from which nanotubes form into a test tube, then adjusting such conditions as temperature and pressure, the nanotubes could organize themselves into one of many different configurations, several never seen previously.
“You could imagine that one type of nanotube forms at 25 degrees Celsius, but another type with very different physical and chemical properties would form at 70 degrees,” Fenniri said. “That’s a simplification, of course, but it illustrates the principles we have uncovered.”
The relative ease of manipulating the properties of nanotubes makes Fenniri optimistic that many new applications will be possible. One possibility is to use the nanotubes in disease treatment.
“Many drugs destroy infectious bacteria by poking holes in their cellular membranes and leaking out their nutrients, just like pricking a hole in a balloon,” he said. “Our nanotubes could also act in this manner, but in addition, they have the ability to lure the bacteria with a bait that guides them to the cell membrane where they can start destroying the cell.”
Further exploitation of the tubes’ dial-up properties could lead to nanotubes that conduct electricity or photons, making them useful in computer memory systems, high-definition displays, biosensors and drug delivery systems. Fenniri is hopeful the findings will prove beneficial in many fields.
“Nanotechnology relies on our ability to control the behavior of matter at the molecular scale,” he said. “The versatility and robustness of our system is already pointing the way towards numerous applications in a fairly broad range of disciplines. It should help nanotubes on their way to becoming the nanoworld’s jack-of-all-trades.”
Funding for this research is provided by grants from the National Science Foundation and the American Chemical Society, awards from 3M and the Research Corp., and by Purdue University.
Writer: Chad Boutin, (765) 494-2081, cboutin@purdue.edu
Source: Hicham Fenniri, (765) 494-5241, hf@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



