Giammar Seeking New Solutions for Underground Carbon Storage
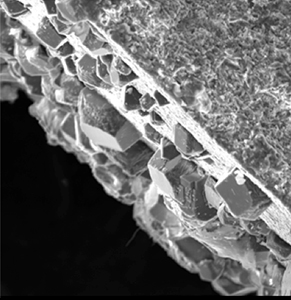
Giammar Scanning electron micrograph that illustrates the precipitation of magnesium carbonate (left side) on the surface of an artificial rock prepared by vacuum sintering of magnesium silicate. The precipitation occurred from the reaction of an aqueous solution equilibrated with a high pressure of carbon dioxide (100 times atmospheric) with the magnesium silicate.
Giammar, professor in energy, environmental & chemical engineering in the School of Engineering & Applied Science, has been working with the Consortium for Clean Coal Utilization (CCCU) since its inception to find ways to reuse or safely store emissions or waste from coal-fired power plants, including fly ash or carbon dioxide. This ties in well with his research, which focuses on chemical reactions that affect the outcome and transport of heavy metals and radionuclides in natural and engineered water systems.
In a new $1.28 million project funded by the U.S. Department of Energy, Giammar is looking at the potential for fractured basalt, a layer of common mineral-rich rock, to store carbon dioxide emissions. He and a team of researchers will work with finger-sized basalt samples in the lab to see how the rock tolerates the transport of carbon dioxide and the chemical reactions that take place among the rock’s natural minerals and the carbon dioxide.
Geologic carbon sequestration, also known as carbon capture and storage, requires deep underground storage that includes porous open space, a permeable material and an impermeable cap so that the CO2 doesn’t leak out. Many current methods of geologic carbon sequestration use sandstone, an abundant porous and permeable material. But CO2 remains as a separate phase of either a gas or supercritical fluid within sandstone, creating the potential for leaks.
However, when CO2 is injected into basalt through the rock’s fractures, which are naturally created cracks caused by high pressure or temperatures, it reacts with the calcium, magnesium and iron within the basalt to create carbonate minerals, a solid product without potential to leak.
Giammar said there are three potential outcomes to this process.
“As you convert the minerals and basalt into carbonate minerals, there is a volume expansion, so you could fill up the fractures and seal off the system from further reactions, making it self-limiting, which is the worst-case scenario,” he said. “The best-case scenario would be that this volume expansion starts to exert stresses on the rocks and opens up new fractures, so it could be self-propagating.”
But, he said, there could also be something in between, with some fracturing of rocks and some filling or partial filling of prior fractures.
“We’ve got a whole range of possibilities,” he said. “We don’t really know the answer to this. Whether or not basalts will be tenable formations for sequestration is critical.”
Giammar will be working with Washington University colleagues Mark Conradi, PhD, professor of physics; Sophia Hayes, PhD, associate professor of chemistry; and Philip Skemer, PhD, assistant professor of earth & planetary sciences, all colleagues from previous CCCU-supported research projects, as well as with Brian Ellis, assistant professor of civil and environmental engineering at the University of Michigan.
Conradi and Hayes will apply their expertise in nuclear magnetic resonance (NMR), which allows researchers to look at chemical reactions as they occur in real time at high temperatures and pressures similar to those that occur inside the earth to measure the progress. Skemer, an expert in the physical properties of rock, will create artificial basalt in the lab to be used alongside the natural basalt fragments. Ellis specializes in building reactors that supply a confining pressure to rocks and push fluids up through them.
Giammar also has completed three prior research projects in collaboration with the CCCU. One looked at the rates of reactions of CO2, minerals and water. The second was a steppingstone to the current project, looking at the transport and reactions of CO2, minerals and water, but using powder instead of rocks, and the third studied the fate of metals in fly ash from coal combustion.
In addition to the research collaborations with colleagues at WUSTL, Giammar has developed an international collaboration with Anurag Mehra, PhD, and graduate students at the Indian Institute of Technology (IIT) Bombay.
“They look at the same types of systems but at lower pressures and have done some nice parallel experiments,” Giammar said. “The project has benefited from this international collaboration.”
Media Contact
All latest news from the category: Ecology, The Environment and Conservation
This complex theme deals primarily with interactions between organisms and the environmental factors that impact them, but to a greater extent between individual inanimate environmental factors.
innovations-report offers informative reports and articles on topics such as climate protection, landscape conservation, ecological systems, wildlife and nature parks and ecosystem efficiency and balance.
Newest articles

Nerve cells of blind mice retain their visual function
Nerve cells in the retina were analysed at TU Wien (Vienna) using microelectrodes. They show astonishingly stable behavior – good news for retina implants. The retina is often referred to…

State-wide center for quantum science
Karlsruhe Institute of Technology joins IQST as a new partner. The mission of IQST is to further our understanding of nature and develop innovative technologies based on quantum science by…

Newly designed nanomaterial
…shows promise as antimicrobial agent. Rice scientists develop nanocrystals that kill bacteria under visible light. Newly developed halide perovskite nanocrystals (HPNCs) show potential as antimicrobial agents that are stable, effective…



