Hierarchically-Porous Polymers with Fast Absorption
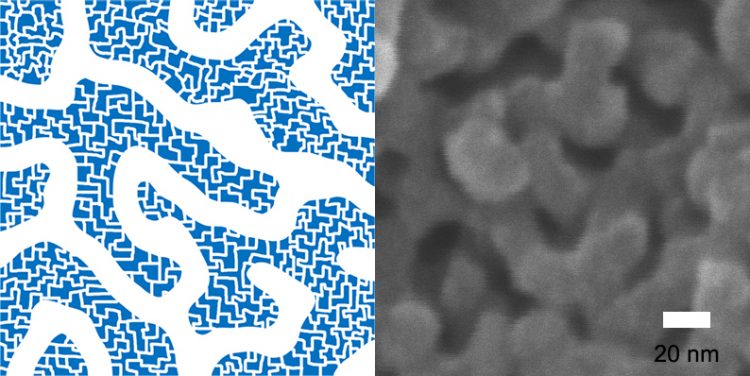
Figure 1 – Net-like Structure of Hierarchically-Porous Polymers with Mesopores and Micropores on the walls of Mesopores.
Professor Myungeun Seo and his research team from the Graduate School of Nanoscience and Technology at KAIST has developed a method to form micropores of less than 2 nanometers within porous polymers where 10 nanometers long mesopores connect like a net. The advantage of the porous polymers is fast absorption of molecules.
Porous polymers with micropores of less than 2 nanometers, like a zeolite, have a large surface area. They are used as a means to store hydrogen-based molecules or as a catalytic support that can be used as a surface to convert a material into a desired form. However, because the size of the pores in its path was too small for the molecules, it took a long time to spread into the pores and reach the surface.
To reach the surface efficiently, a lung cell or the vein of a leaf has a structure wherein the pores are subdivided into different sizes so that the molecule can spread throughout the organ. A technology that can create not only micropores but also bigger pores was necessary in order to create such structure.
The research team solved the issue by implementing a “self-assembly” of block polymers to easily form a net-like nanostructure from mesopores of 10 nanometers.
The team created hierarchically-porous polymers consisting of two different types of pores by using a hypercrosslinking reaction along with the “self-assembly” method. The reaction creates micropores within the chain after the polymer chain is confined by a chemical bond.
This porous polymer has micropores that are smaller than 2 nanometers on the walls of mesopores while 10 nanometers long mesopores forming 3-dimensional net structures. Because of the “self-assembly” method, the size of mesopores can be adjusted within the range of 6 to 15 nanometers.
This is the first case where a porous polymer has both well-defined mesopores and micropores. The research team verified the effect of hierarchically-porous structures on absorption of molecules by confirming that the porous polymer had faster absorption speeds than a polymer consisting only of micropores.
Professor Seo said, “The study has found a simple way to create different sizes of pores within a polymer.” He expected that the hierarchically-porous polymers can be used as a catalytic support in which fast diffusion of molecules is essential, or for molecule collection.
The research was sponsored by National Research Foundation of Korea and published online in the Journal of the American Chemical Society.
Associated links
KAIST article
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



