Transport molecule forms a protective structure to guide proteins to cell membrane
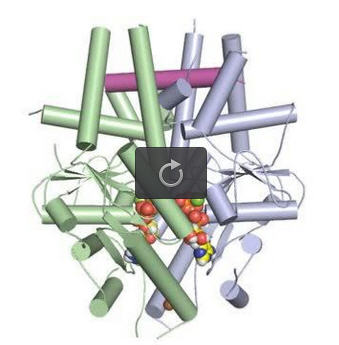
A tail-anchored membrane protein (magenta) bound to the ATP-bound (spheres) Get3 dimer (green and blue). Credit: Robert Keenan, University of Chicago
This structure shields tail-anchored membrane proteins – which have roles in a wide variety of cellular functions from neurotransmitter release to insulin production – from harmful aggregation or misfolding as they move through the inner environment of a cell. The findings clarify the mechanism behind a fundamental biological process.
“The cell is able to shield tail-anchored proteins and get them to the right membrane at the right time through this two-subunit complex,” said study co-senior author Robert Keenan, PhD, associate professor of biochemistry and molecular biophysics at the University of Chicago. “The simple analogy is that it's like a hot dog bun. It presents a large groove that accommodates the transmembrane region of the protein that's being targeted.”
Membrane proteins are involved in a number of essential cellular functions such as signaling, catalyzing chemical reactions, and nutrient and ion transport. These proteins contain hydrophobic regions that allow them to be embedded in the hydrophobic lipid bilayer of cell membranes.
During protein synthesis, which takes place inside the aqueous environment of a cell, emerging hydrophobic regions are predisposed toward clumping together in order to avoid water. As aggregation can be harmful, protective mechanisms immediately shield these regions as they emerge and then chaperone proteins to the membrane.
Unlike the majority of membrane proteins, tail-anchored proteins contain only one hydrophobic region that is usually the last to be synthesized. Because of this, the coordination of several molecular factors – together comprising the GET ('guided entry of tail-anchored proteins') pathway – are required to prevent tail-anchored proteins from aggregating.
The key component of this pathway is a targeting factor known as Get3, which captures and shields the hydrophobic region of tail-anchored proteins. The mechanism by which Get3 performs its protective function has been debated, leaving the study of the entire pathway in flux.
Previous studies have argued that four molecules of Get3 combine to form a tetrameric complex that encase its cargo. But other studies, including ones from Keenan and co-author Ramanujan Hedge, PhD, of the Medical Research Council in Cambridge, have pointed to a dimeric complex involving only two subunits of Get3.
To resolve this question, the team, led by Agnieszka Mateja, PhD, postdoctoral fellow at the University of Chicago, first assembled Get3-substrate complexes in the laboratory. Using purified components to mimic physiological conditions, they found dimeric structures.
Get3 has been shown to form dimers when linked to ATP, but Get3 also naturally degrades ATP over time, which likely led to previous observations of a tetrameric Get3-substrate complexes under non-physiological conditions. When the researchers expressed a tail-anchored protein in bacteria with a modified version of Get3 that was unable to degrade ATP, only dimeric complexes were formed.
To visualize the physiologically-relevant structure, the researchers used protein crystallization to study Get3 while it was holding a hydrophobic tail-anchored protein. In collaboration with Tony Kossiakoff, PhD, professor of biochemistry and molecular biophysics at the University of Chicago, the team designed synthetic antibody fragments that bound to specific parts of the Get3 dimer.
These antibodies facilitated packing of Get3 and the substrate into the crystal. Analyzing this complex atom by atom, the team found that Get3 functioned exactly as they had predicted – with two Get3 subunits protecting the tail-anchored protein in a large hydrophobic groove.
“There was a lot of painstaking engineering that went into this, but ultimately we defined the structure of the physiologic complex,” Keenan said. “It's a very important biological pathway, a hard technical problem, and now only the second instance where we have a glimpse of how a hydrophobic transmembrane protein binds to one of its targeting factors. This is a fundamental insight into how a cell works.”
With the structure solved and the Get3 protective mechanism clarified, Keenan and his team are now investigating the mechanism of other steps in the GET pathway.
“The mechanistic details of the GET pathway are different if you assume a dimer model or a tetramer model,” Keenan said. “By demonstrating that the physiologically relevant targeting complex is dimeric, we get a relatively simple, elegant working model. It really clarifies thinking in this field.”
###
The study, “Structure of the Get3 targeting factor in complex with its membrane protein cargo,” was supported by the National Institutes of Health, the US Department of Energy, the UK Medical Research Council and the Chicago Biomedical Consortium. Additional authors include Marcin Paduch, Hsin-Yang Chang, Anna Szydlowska, and Ramanujan S.Hegde.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



