Weizmann Institute Scientists Regenerate Heart Cells in Mice
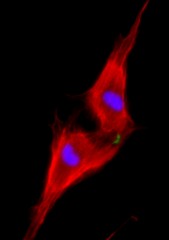
Weizmann Institute of Science Two neonatal cardiomyocytes (stained red) undergoing cell division after treatment with NRG1.
When a heart attack strikes, heart muscle cells die and scar tissue forms, paving the way for heart failure. Cardiovascular diseases are a major cause of death worldwide, in part because the cells in our most vital organ do not get renewed.
As opposed to blood, hair, or skin cells that can renew themselves throughout life, our heart cells cease to divide shortly after birth, with very little renewal in adulthood. New research at the Weizmann Institute of Science provides insight into the question of why the mammalian heart fails to regenerate, and also demonstrated, in adult mice, the possibility of turning back this fate. This research appeared on April 13 in Nature Cell Biology.
Prof. Eldad Tzahor of the Institute’s Department of Biological Regulation thought that part of the answer to the regeneration puzzle might lie in his area of expertise: embryonic development, especially of the heart. Indeed, it was known that a protein called ERBB2 – which is well studied because it can pass along growth signals promoting certain kinds of cancer – plays a role in heart development.
ERBB2 is a specialized receptor – a protein that transmits external messages into the cell. It generally works together with a second, related receptor by binding a growth factor called neuregulin 1 (NRG1) to transmit its message. NGR1 is already being tested in clinical studies as a treatment for heart failure.
Dr. Gabriele D’Uva, a postdoctoral fellow in Prof. Tzahor’s research group, wanted to know exactly how NRG1 and ERBB2 are involved in heart regeneration. In mice, new heart muscle cells can be added for up to a week after birth; in fact, newborn mice can regenerate damaged hearts, while seven-day-old mice cannot.
Dr. D’Uva and research student Alla Aharonov observed that heart muscle cells called cardiomyocytes that were treated with NRG1 continued to proliferate on the day of birth, but that the effect dropped dramatically within a week, even with ample amounts of NRG1. Further investigation showed that the difference between a day and a week was the amount of ERBB2 on the cardiomyocyte membranes.
The team then created mice in which the gene for ERBB2 was “knocked out” in cardiomyocytes. This had a severe impact: the mice had hearts with walls that were thin and balloon-like – a cardiac pathology known as dilated cardiomyopathy. The conclusion was that cardiomyocytes lacking ERBB2 do not divide, even in the presence of NRG1.
Next, the team reactivated the ERBB2 protein in adult mouse heart cells, in which cardiomyocytes normally no longer divide. This resulted in extreme cardiomyocyte proliferation and hypertrophy – excessive growth and development of the individual cardiomyocytes – leading to a giant heart (cardiomegaly) that left little room for blood to enter. Says Prof. Tzahor: “Too little or too much of this protein had a devastating impact on heart function.”
The question then became: if one could activate ERBB2 for just a short period in an adult heart following a heart attack, might it be possible to get the positive results, i.e., cardiac cell renewal, without negative ones such as hypertrophy and scarring?
Testing this idea, the team found that they could, indeed, activate ERBB2 in mice for a short interval only following an induced heart attack, and obtain nearly complete heart regeneration within several weeks. “The results were amazing,” says Prof. Tzahor. “As opposed to extensive scarring in the control hearts, the ERBB2-expressing hearts had completely returned to their previous state.”
Investigation of the regenerative process through live imaging and molecular studies revealed how this happens: the cardiomyocytes “de-differentiate” – that is, they revert to an earlier form, something between an embryonic and an adult cell, which can then divide and differentiate into new heart cells. In other words, the ERBB2 took the cells back a step to an earlier, embryonic form; and then stopping its activity promoted the regeneration process.
In continuing research, Prof. Tzahor and his team began to outline the pathway – the other proteins that respond to the NRG1 message inside the cell. “ERBB2 is clearly at the top of the chain. We have shown that it can induce cardiac regeneration on its own. But understanding the roles of the other proteins in the chain may present us with new drug targets for treating heart disease,” says Dr. D’Uva.
Prof. Tzahor points out that clinical trials of patients receiving the NRG1 treatment might not be overly successful if ERBB2 levels are not boosted as well. He and his team plan to continue researching this signaling pathway to suggest ways of improving the process, which may, in the future, point to ways of renewing heart cells.
Because this pathway is also involved in cancer, well-grounded studies will be needed to understand exactly how to direct the cardiomyocyte renewal signal at the right place, the right time, and in the right amount. “Much more research will be required to see if this principle could be applied to the human heart, but our findings are proof that it may be possible,” he says.
Participating in this research were Profs. Yosef Yarden and Michal Neeman, also of the Department of Biological Regulation. In addition, Prof. Jonathan Leor of Chaim Sheba Medical Center, Israel, and Prof. Richard P. Harvey of the University of South Wales, Australia, contributed to this research.
Prof. Eldad Tzahor’s research is supported by the Louis and Fannie Tolz Collaborative Research Project; the European Research Council; and the estate of Jack Gitlitz.
The Weizmann Institute of Science in Rehovot, Israel, is one of the world’s top-ranking multidisciplinary research institutions. The Institute’s 3,800-strong scientific community engages in research addressing crucial problems in medicine and health, energy, technology, agriculture, and the environment. Outstanding young scientists from around the world pursue advanced degrees at the Weizmann Institute’s Feinberg Graduate School. The discoveries and theories of Weizmann Institute scientists have had a major impact on the wider scientific community, as well as on the quality of life of millions of people worldwide.
Contact Information
Jennifer Manning
Director, Science Content
jennifer@acwis.org
Phone: 212-895-7952
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



