Faster, not stronger: How a protein regulates gene expression
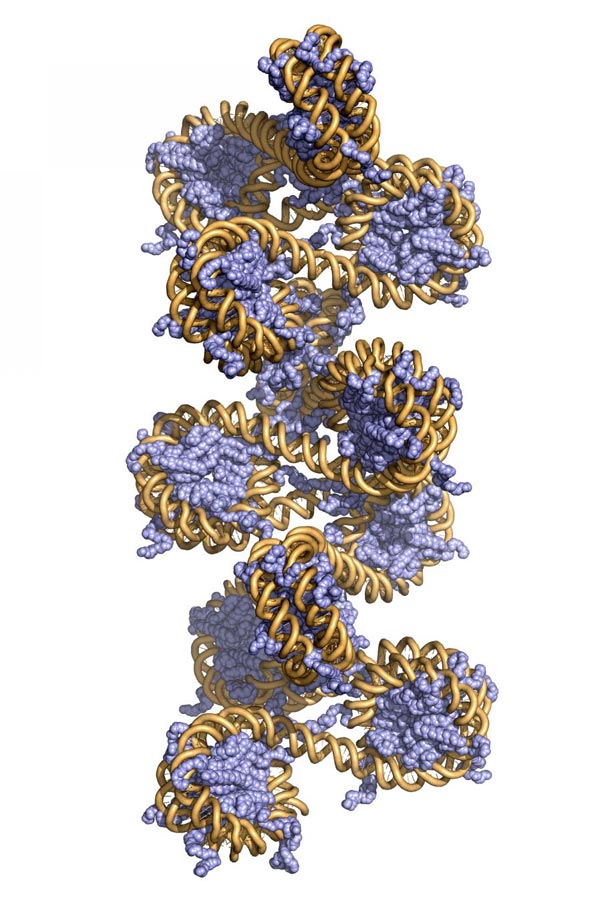
This is a 3-D model of chromatin. Credit: Beat Fierze/EPFL
Inside the cell, DNA is tightly coiled and packed with several proteins into a structure called “chromatin”, which allows DNA to fit in the cell while also preventing genes from being expressed at the wrong time.
Guided by a chemical “barcode”, specialized effector proteins can bind chromatin and either unwind it or compact further to activate or silence genes. This system has enormous implications for biology and medicine, e.g. cancer research.
However, the efficiency of effector-chromatin interactions have been elusive, especially given the weak binding between the two. Tracking these interactions one molecule at a time, EPFL scientists have shown for the first time how a major effector protein speeds up its search for chromatin binding sites pairing up with others of its kind. The elegant molecular mechanism is published in Nature Communications.
Packaging DNA
Every human cell contains an astonishing 1.8 meters of DNA. In order to fit, DNA is tightly wrapped around spool-shaped proteins, the histones, which package it down to 0.09 millimeters – a 20,000-fold reduction in length. The histone-DNA complex is called chromatin.
Through it, the cell can regulate which genes are active at any given time by unwinding that part of packed DNA. Conversely, the cell can “repress” genes by forming compact and dense chromatin fibers. All this is done by “effector” proteins, which attach to chromatin and change their 3D structure to either open or compact DNA containing the gene of interest.
This mechanism is explored by a host of biological and medical fields, e.g. genetics and cancer research, as it is the key to regulating genes in a cell. However, there are gaps in our understanding of how such a crucial system remains efficient, given that the binding between effectors and histones is too weak. Several theories have been proposed, but the problem has persisted.
Faster, not stronger
The lab of Beat Fierz at EPFL has now shown that a major effector protein increases its efficiency not by binding chromatin more strongly, but rather by binding and re-binding it faster. The protein, called HP1α, dissociates from chromatin relatively easily, something that is common across proteins that interact with DNA. To make up for this “loose binding”, HP1α speeds up its binding rate, but also gets together in pairs to maximize its binding sites.
The scientists used single-molecule measurements, a highly sensitive experimental method. The technique, never used in this context before, allowed the researchers to observe individual HP1α proteins interact with chromatin in real time. The team also synthesized chromatin fibers that contained the appropriate chemical identifiers, and they used this system to explore HP1α binding under different conditions and experimental parameters.
Along with the increase in binding rate, the scientists also found that when HP1α connects to other HP1α proteins to make dimers, it increases its binding sites so that it can maximize its interaction with chromatin. It is important to remember here that interactions inside the cell are not static; molecules connect and disconnect constantly, often competing for the same site. By increasing its binding speed and multiplying its binding site, HP1α has a better chance of binding chromatin for longer and thus regulating gene expression.
Given the importance of this mechanism to cell proliferation and growth, it holds particular significance in cancer research, and potentially other diseases caused by problematic gene regulation. Fierz's group is now working on extending their single-molecule methodology to study more complex processes, in particular how damaged DNA is repaired. “We want to observe complex biology as it happens, and understand it on a quantitative level,” he says. “Our chemical methods give us complete control over protein-chromatin dynamics, and the current study sets the stage for such unprecedented insights.”
###
Reference
Kilic S, Bachmann AL, Bryan LC, Fierz B. Multivalency governs HP1α association dynamics with the silent chromatin state. Nature Communications 18 June 2015. DOI: 10.1038/ncomms8313
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



