Aromatic couple makes new chemical bonds
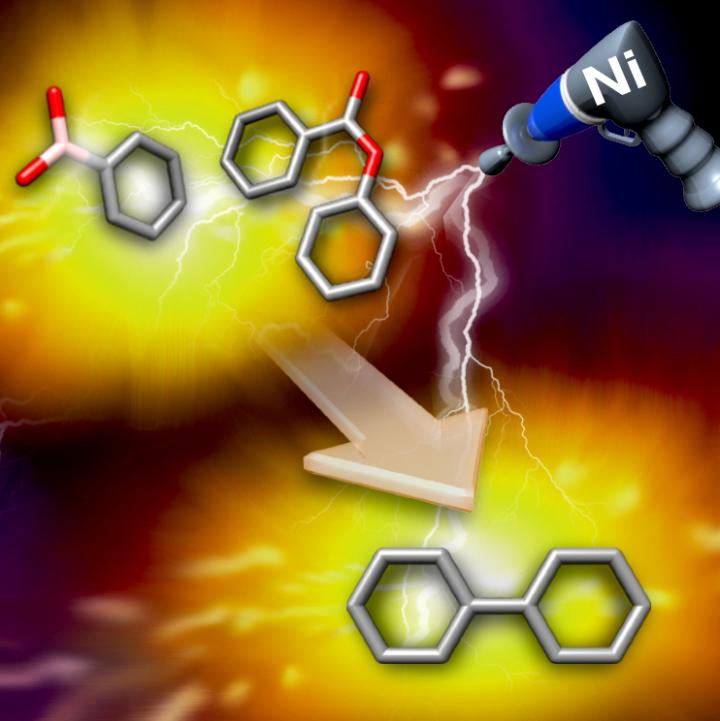
A nickel catalyst triggers the cross-coupling reaction between aromatic esters and boronic acids. Credit: ITbM, Nagoya University
Esters have been identified to act as a new and clean coupling partner for the carbon-carbon bond forming cross-coupling reaction to make useful compounds for pharmaceuticals, agrochemicals and organic materials.
In a new collaborative study published online in Nature Communications, synthetic and theoretical chemists at the Institute of Transformative Bio-Molecules (ITbM), Nagoya University and the NSF Center for Selective C-H Functionalization (NSF-CCHF) at Emory University have shown that a relatively inexpensive nickel catalyst triggers the decarbonylative cross-coupling between aromatic esters and boronic acids.
This cross-coupling reaction, known as the Suzuki-Miyaura cross-coupling reaction is an extremely powerful strategy to synthesize a variety of significant organic compounds on an industrial scale, thus leading to the Nobel Prize in Chemistry in 2010. The Suzuki-Miyaura cross-coupling reaction is usually catalyzed by a palladium catalyst to couple boronic acids with organic halides. However, while this reaction is very powerful these reaction partners can generate corrosive waste byproducts. Hence, many groups have been working to develop alternative coupling partners.
Researchers at ITbM and CCHF have demonstrated that readily available esters can be used to generate useful cross-coupled products, including the derivatives of plant metabolites and of a drug for treating hypertension, telmisartan.
Esters are carbonyl compounds found in fats and oils, and are known for their distinctive odors such as in bananas and pineapples, thus making them a popular ingredient in perfumes. Due to their high commercial and synthetic accessibility, the use of esters to couple with boronic acids in the Suzuki-Miyaura cross-coupling greatly expands the scope of available substrates for the reaction.
“From its low cost and our extensive experience, we chose to use nickel as a catalyst for developing the decarbonylative cross-coupling reaction with esters,” says Kenichiro Itami, Center-Director and Professor at ITbM. “Through screening of various conditions, we were pleased to find that a catalytic amount of nickel acetate along with sodium carbonate as a base is effective for the cross-coupling reaction of aromatic esters with boronic acids, to obtain the desired the decarbonylative products in good yield.”
Nickel acetate is approximately 300 times less expensive compared to palladium acetate and proved to be active on the gram-scale cross-coupling between aromatic esters and boronic acids. In addition, a range of aromatic ester substrates was compatible in the reaction and no side products from carbonylative coupling were observed.
This chemoselective cross-coupling reaction has also proved to be applicable towards the synthesis of flavone and its derivatives, and has also been utilized to functionalize a phenyl ester derivative of telmisartan, a drug molecule with a relatively complex structure.
Theoretical calculations conducted by Professor Djamaladdin Musaev of Emory University provided valuable insight to the mechanism of the decarbonylative cross-coupling reaction between aromatic esters and boronic acids. The role of the base and the order of the reaction steps were identified by density functional theory calculations, indicating that the decarbonylation step occurs after transmetalation, which is accelerated by the presence of the base.
“This is a beautiful work in the frontier of organic transformations, and establishes a novel, relatively inexpensive and environmentally friendly synthetic strategy for carbon-carbon bond formation. The accompanied computational efforts revealed key mechanistic details of this novel reaction. I am grateful to Professor Itami and the entire ITbM team for providing the opportunity for us to contribute to this novel research project. This work is part of our ongoing, highly enjoyable and productive collaborations, and we thank ITbM (Nagoya University) and NSF-CCHF (Emory University), as well as MEXT, JSPS (Japan) and NSF (USA) for their support of our far-reaching international collaborative efforts,” says Musaev.
“We were extremely fortunate to be able to work with Professor Musaev to look into the mechanism of this unusual decarbonylative cross-coupling reaction,” says Itami. “This is obviously a result of the strong collaboration between ITbM and CCHF. We will continue to work together to conduct further optimization of the reaction system to expand its scope, including aliphatic esters.”
Itami and his colleagues envisage that ongoing advances in this reaction method may lead to the development of new industrial processes for making biologically and structurally active compounds.
###
This article “Decarbonylative organoboron cross-coupling of esters by nickel catalysis” by Kei Muto, Junichiro Yamaguchi, Djamaladdin G. Musaev & Kenichiro Itami is published online on June 29, 2015 in Nature Communications.
DOI: 10.1038/ncomms8508
About WPI-ITbM
The World Premier International Research Center Initiative (WPI) for the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. As part of the Japanese science ministry's MEXT program, ITbM aims to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a “Mix-Lab” style, where international young researchers from multidisciplinary fields work together side-by-side in the same lab. Through these endeavors, ITbM will create “transformative bio-molecules” that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
About NSF-CCHF
The NSF Center for Chemical Innovation on Selective C-H Functionalization is comprised of 23 collaborating research groups located at 15 universities within the US. The goal of the Center is to leverage its collaborative potential to develop technology for selective C-H functionalization that will revolutionize the practice and reshape the teaching of chemical synthesis, empowering end users in materials science, fine chemicals development, and drug discovery.
About The Virtual Institute for C-H Functionalization (VICHF)
The Virtual Institute for C-H Functionalization (VICHF) has been established to act as a collaborative forum for international research partners to encourage the exchange of ideas and students and to harbor a global research community for this developing field of chemistry.
Author Contact
Professor Kenichiro Itami
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-788-6098
E-mail: itami@chem.nagoya-u.ac.jp
Professor Djamaladdin G. Musaev
Cherry L. Emerson Center for Scientific Computation
Center for Chemical Innovation on Selective C-H Functionalization
Emory University
TEL: +1- 404-727-2382 FAX: +1-404-727-7412
E-mail: dmusaev@emory.edu
Media Contact
Dr. Ayako Miyazaki
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-4999 FAX: +81-52-789-3240
E-mail: press@itbm.nagoya-u.ac.jp
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
E-mail: kouho@adm.nagoya-u.ac.jp
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



