Eliminating entanglements
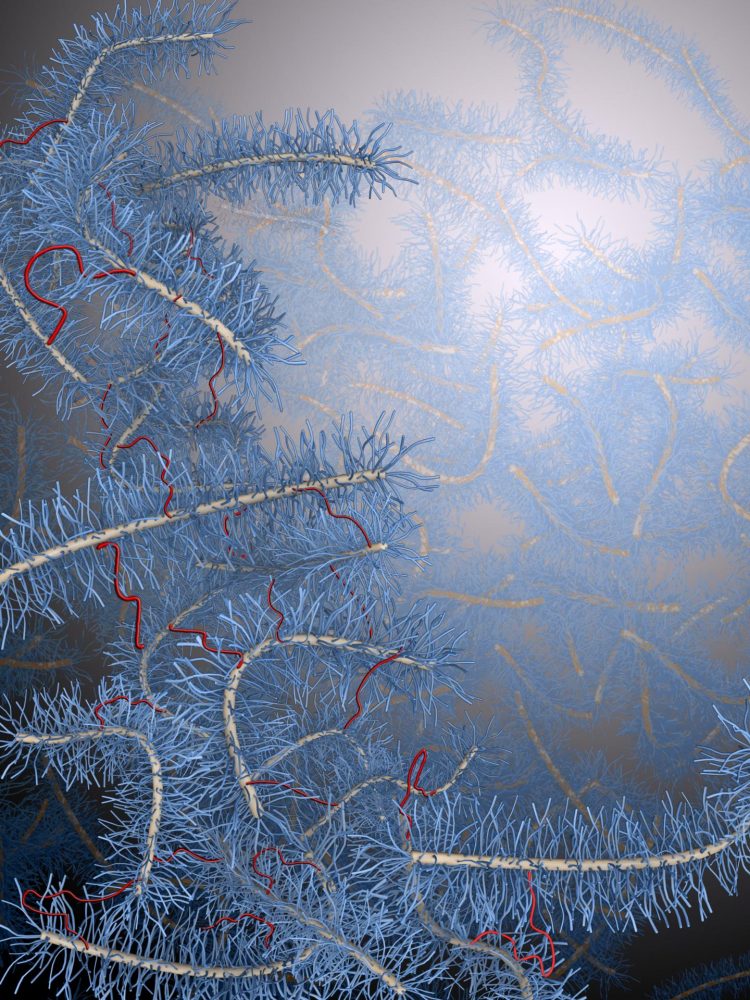
This is an ultra-soft elastomer fabricated by crosslinking bottlebrush polymers contains only crosslinks (red chains) and no entanglements. (Image courtesy of Li-Heng Cai, Harvard SEAS.)
Medical implants mimic the softness of human tissue by mixing liquids such oil with long silicone polymers to create a squishy, wet gel. While implants have improved dramatically over the years, there is still a chance of the liquid leaking, which can be painful and sometimes dangerous.
Now, led by David A. Weitz, Mallinckrodt Professor of Physics and Applied Physics at Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) and associate faculty member at the Wyss Institute for Biologically Inspired Engineering at Harvard, a team of polymer physicists and chemists has developed a way to create an ultra-soft dry silicone rubber. This new rubber features tunable softness to match a variety of biological tissues, opening new opportunities in biomedical research and engineering.
The material is featured on the cover of the journal Advanced Materials.
“Conventional elastomers are intrinsically stiff because of how they are made,” said lead author Li-Heng Cai, a postdoctoral fellow at SEAS. “The network strands are very long and are entangled, similar to a bunch of Christmas lights, in which the cords are entangled and form knots. These fixed entanglements set up an intrinsic lower limit for the softness of conventional elastomers.”
In order to fabricate a soft elastomer, the team needed to eliminate the entanglements from the beginning by developing a new type of polymer that was fatter and less prone to entanglement than linear polymers. The polymers, nicknamed bottlebrushes, are easily synthesized by mixing three types of commercially available linear silicone polymers.
“Typically the fabrication of such bottlebrush molecules requires complex chemical synthesis,” said co-first author Thomas E. Kodger, Ph.D.' 2015, now a postdoctoral fellow at University of Amsterdam. “But we found a very simple strategy by carefully designing the chemistry. This system creates soft elastomers as easily as silicone kits sold commercially.”
The softness of the elastomers can be precisely controlled by adjusting the amount of cross-linked polymers to mimic everything from soft brain tissue and relatively stiff cells.
“If there are no crosslinks, all the bottlebrush molecules are mobile and the material will flow like a viscous liquid such as honey,” said Cai. “Adding crosslinks connects the bottlebrush molecules and solidifies the liquid, increasing the material stiffness.”
In addition to controlling the softness, the team also found a way to independently control the liquid-like behavior of the elastomer.
“To make the conventional elastomer softer, one needs to swell it in a liquid,” said coauthor Michael Rubinstein, John P. Barker Distinguished Professor in Chemistry at the University of North Carolina at Chapel Hill. “But now we can adjust the length of 'hairy' polymers on the bottlebrush molecules to tune the liquid-like behavior of soft elastomers — without swelling — allowing us to make these elastomers exceptionally non-adhesive yet ultra-soft.”
These qualities make the material not only ideal for medical devices, such as implants, but also for commercial products such as cosmetics.
“The independent control over both softness and liquid-like behavior of the soft elastomers will also enable us to answer fundamental questions in biomedical research,” said Weitz. “For example, stem cell differentiation not only depends on the softness of materials with which they are in contact, but recent findings suggest that it is also affected by how liquid-like the materials are. This discovery will provide entirely new materials to study the cell behavior on soft substrates.”
“The exceptional combination of softness and negligible adhesiveness will greatly broaden the application of silicon-based elastomers in both industry and research,” said Weitz.
###
In addition to his role on the faculty at SEAS, Weitz is the director of Harvard's Materials Research Science and Engineering Center, co-director of the BASF Advanced Research Initiative, a member of the Kavli Institute for Bionano Science and Technology, and an Associate Faculty Member at the Wyss Institute for Biologically Inspired Engineering.
In addition to Cai, Kodger, Weitz, and Rubinstein, coauthors included Rodrigo E. Guerra, Ph.D.' 2015, now a postdoctoral fellow at New York University; and Adrian F. Pegoraro, a postdoctoral fellow at SEAS.
This research was supported in part by the National Science Foundation (DMR-1310266) and the Harvard Materials Research Science and Engineering Center (DMR-1420570).
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



