New technology can expand LED lighting, cutting energy use and greenhouse gas emissions
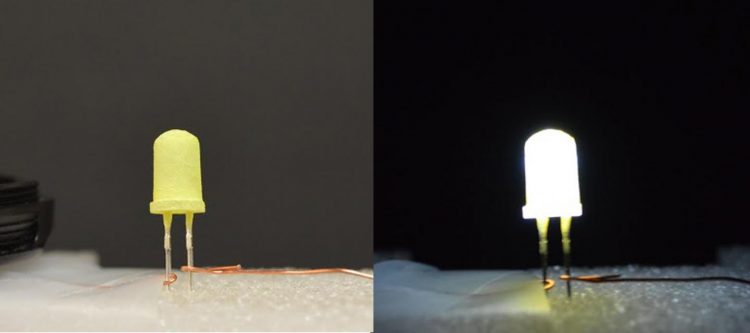
An LED coated with a yellow "phosphor" is shown turned off (left) and then turned on (right). This "green" LED is inexpensive and provides warm white light. Credit: Zhichao Hu, Ph.D.
The scientists will discuss their research at the 250th National Meeting & Exposition of the American Chemical Society (ACS). ACS is the world's largest scientific society. The national meeting, which takes place here through Thursday, features more than 9,000 presentations on a wide range of science topics.
“If more people in the U.S. used LEDs in their homes and businesses, the country's electricity consumption could be cut in half,” says Zhichao Hu, Ph.D., a member of the Rutgers University team that performed the research under the direction of Jing Li, Ph.D.
At that time, he was a graduate student. He is now a postdoc at Rutgers and is studying the recovery of rare-earth elements there. Zhichao adds that studies show substituting one LED light for a common incandescent light bulb in every American household could save the nation $700 million annually in energy costs.
To achieve the common, soft white light that consumers expect, current LED technologies typically use a single semiconductor chip to produce light, usually blue, and then rely on a yellow-emitting “phosphor” coating to shift the color to white.
That's because LEDs do not emit a white light. The phosphor is made from materials, such as cerium-doped yttrium aluminum garnet, that are composed of rare-earth elements. These elements are expensive and in limited supply, since they are primarily available only from mining operations outside the U.S. Additionally, the light output of these phosphors tends to be harsh, “cold” colors.
Li's team is developing hybrid phosphor-based technologies that are much more sustainable, efficient and low-cost. They combine common, earth-abundant metals with organic luminescent molecules to produce phosphors that emit a controllable white light from LEDs.
By varying the metal and organic components, the researchers can systematically tune the color of the phosphors to regions of the visible light spectrum that are most acceptable to the human eye, Hu and Li note. The team is continuing to experiment and develop other rare-earth-free LED phosphors based on different metals and organic compounds.
Many material combinations are possible, so they use a computational approach to initially sort through the possibilities and to predict what color of light the various metals and organics combinations will emit. They then test the best combinations experimentally.
Their approach allows a systematic fine tuning of band gaps and optical emissions that cover the entire visible range, including yellow and white colors. As a result, their LEDs can be fine-tuned to create a warmer white light, similar to cheaper but inefficient incandescent lights. Their approach shows significant promise for use in general lighting applications.
“One of challenges we had to overcome was to figure out the right conditions to synthesize the compound,” Hu notes. “Like cooking, the synthesis requires a 'recipe.' It's often not the case that one can simply mix the starting materials together and get the desired product. We optimized the reaction conditions — temperature and the addition of a solvent — and developed an easy procedure to make the compound with high yield.”
Experiments with some materials have shown that the team's technology can cut LED costs by as much as 90 percent from current methods that rely on rare-earth elements. They have several granted and pending U.S. patents and are exploring manufacturing possibilities.
A press conference on this topic will be held Wednesday, Aug. 19, at 9 a.m. Eastern time in the Boston Convention & Exhibition Center. Reporters may check-in at Room 153B in person, or watch live on YouTube http://bit.
Funding for this research was provided by the National Science Foundation and Rutgers University. Hu is currently funded by the Department of Energy's Critical Materials Institute.
The American Chemical Society is a nonprofit organization chartered by the U.S. Congress. With more than 158,000 members, ACS is the world's largest scientific society and a global leader in providing access to chemistry-related research through its multiple databases, peer-reviewed journals and scientific conferences. Its main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact newsroom@acs.org.
Note to journalists: Please report that this research is being presented at a meeting of the American Chemical Society.
Title A new rare-earth-free hybrid phosphor for efficient solid-state lighting
Abstract Light-emitting diodes (LEDs) have been “in the spot light” recently; this Nobel Prize winning technology is making its way into our homes, offices, and schools as LEDs are rapidly replacing fluorescent and incandescent lights. Phosphor-converted white LEDs (PC-WLEDs) are the most economically viable LEDs for general lighting. A common way to fabricate a PC-WLED is to coat a blue-emitting LED with a yellow phosphor. Current commercially available phosphors predominantly rely on rare-earth elements for their superb emitting abilities. However, the prices of rare-earth elements have increased up to 49 times over the last decade. Aiming at developing new rare-earth-free sustainable phosphors for efficient solid-state lighting, we build a hybrid yellow phosphor by immobilizing a preselected molecular chromophore into a rigid coordination framework. The immobilization of the molecular chromophore transforms the compound's emission further into the yellow region, with increased quantum yield rivaling that of the commercially available YAG:Ce3+. Coating a blue LED with this new phosphor gives rise to a prototype PC-WLED with high luminous efficacy. This new rare-earth-free yellow phosphor is a strong candidate for use in PC-WLEDs.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….

Climate change can cause stress in herring larvae
The occurrence of multiple stressors undermines the acclimatisation strategies of juvenile herring: If larvae are exposed to several stress factors at the same time, their ability to respond to these…

Making high-yielding rice affordable and sustainable
Plant biologists show how two genes work together to trigger embryo formation in rice. Rice is a staple food crop for more than half the world’s population, but most farmers…



