An approach to new antiviral medicines using a combination of genomics and proteomics
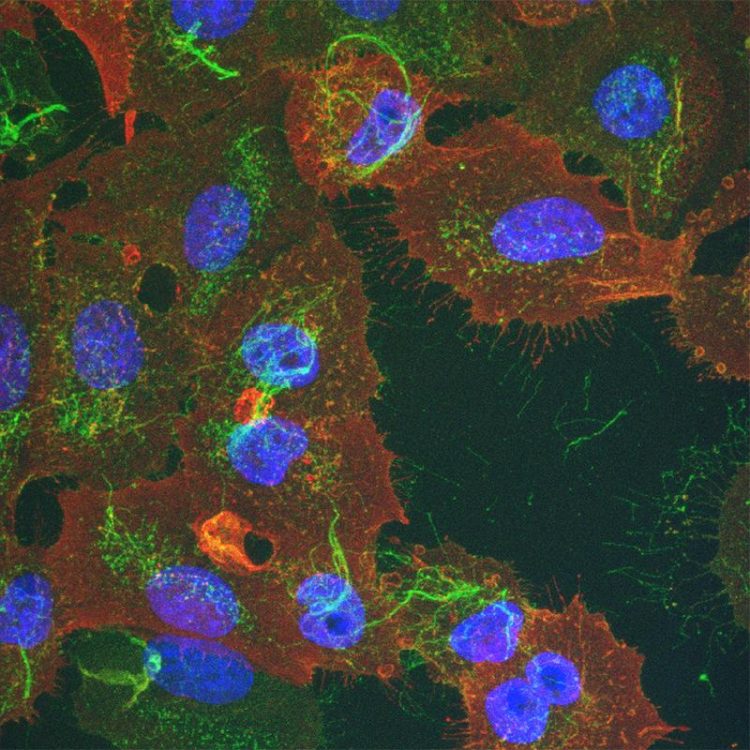
Human lung carcinoma cells infected with influenza A virus (nucleus in blue). The viral proteins haemagglutinin (green) and matrix protein 2 (red) are mainly located on the plasma membrane. Source: Center for Microscopy and Image Analysis, University of Zurich
Between two and ten million people are infected with influenza viruses, that cause seasonal annual epidemics of disease. Influenza viruses are subdivided into influenza A, B, and C and their various subtypes. Influenza A viruses often constitute the main type of the circulating influenza viruses world-wide.
Influenza viruses undergo rapid genetic changes, therefore, influenza vaccines are adapted to the circulating seasonal influenza viruses each year. Moreover, drugs against influenza viruses, so-called antivirals, are required to treat infected individuals and are needed as a future treatment option for the occurrence of new (influenza) viruses – especially in the event of a pandemic. Meanwhile, however, a great number of influenza A viruses have become resistant to the currently available antiviral medications.
An approach to new effective antivirals against viruses is to inhibit the interaction between the virus and its host, i.e. the affected cells in the infected individual, since viruses use human cell proteins to reproduce and proliferate. A blockage of the interaction between virus proteins and cell proteins could inhibit the reproduction of the viruses, thus treating influenza successfully.
So far, the exact mechanism of interaction of influenza A virus with human cells have not been fully understood. Extensive systems-level data are available worldwide, but are seemingly discordant.
A joint collaborative research effort between four international research groups – including the group of Dr Renate König, head of the research team “Cellular Aspects of Pathogen-Host Interactions” at the Paul-Ehrlich-Institut have succeeded in generating a biochemical “map” of essential influenza A virus/host interactions. They achieved this by combining extensive genomic (relating to genes) and proteomic (relating to proteins) data analyses.
With the help of this map, the scientists identified UBR4 (ubiquitin protein ligase E3 component n-recognin 4), a protein in human cells which is responsible for “budding”, i.e. separating the viruses from the cell membrane, the exiting of the pathogen from the cell and subsequent proliferation within and outside the body. The scientists assume that UBR4 is “borrowed” by the virus to deactivate a protection mechanism in humans using its enzymatic function.
According to the scientists, this protection mechanism is as yet not known in detail. The scientists assume that it causes degradation of the viral proteins thus stopping them from being transported to the cell membrane. Based on this model, the influenza A virus thus buys itself a safe passage to the cell membrane. A novel approach to developing new antiviral drugs against the influenza A virus could be to strengthen the above mentioned protective mechanism.
“One advantage of active substances acting on cellular (human) proteins essential for the virus is the fact that the human genome does not constantly change so that active substances should also show long-term efficacy”, as Dr. König explains. Another advantage is that drugs that target cellular proteins may be effective against quite different viruses which utilize the same human protein machinery, of course, without disrupting essential cell functions. And finally, cellular proteins as target structures have the potential to be used to enhance the efficacy of vaccines.
Original publication
Tripathi S, Pohl MO, Zhou Y, Rodriguez-Frandsen A,, Wang G, Stein DA, Moulton HM, DeJesus P, Che J, Mulder LC, Yángüez E, Andenmatten D, Pache L, Manicassamy B, Albrecht RA, Gonzalez MG, Nguyen Q, Brass A, Elledge S, White M, Shapira S, Hacohen N, Karlas A, Meyer TF, Shales M, Gatorano A, Johnson JR, Jang G, Johnson T, Verschueren E, Sanders D, Krogan N, Shaw M, König R#, Stertz S#, García-Sastre A#, Chanda SK# (2015):
Meta- and Orthogonal Integration of Influenza ‘OMICs’ Data Reveals UBR4 as a Critical Regulator of M2 Ion Channel Membrane Trafficking. Cell Host Microbe. 2015 18: 723-735.
# Co-senior authors
The Paul-Ehrlich-Institut, the Federal Institute for Vaccines and Biomedicines, in Langen near Frankfurt/Main is a senior federal authority reporting to the Federal Ministry of Health (Bundesministerium für Gesundheit, BMG). It is responsible for the research, assessment, and marketing authorisation of biomedicines for human use and immunological veterinary medicinal products. Its remit also includes the authorisation of clinical trials and pharmacovigilance, i.e. recording and evaluation of potential adverse effects. Other duties of the institute include official batch control, scientific advice and inspections. In-house experimental research in the field of biomedicines and life science form an indispensable basis for the manifold tasks performed at the institute. The Paul-Ehrlich-Institut, with its roughly 800 members of staff, also has advisory functions nationally (federal government, federal states (Länder)), and internationally (World Health Organisation, European Medicines Agency, European Commission, Council of Europe etc.).
Publication Abstract
http://www.pei.de/EN/information/journalists-press/press-releases/2015/20-approa… – This press release on the PEI-Website
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



