Mystery surrounding non-platinum catalysts for fuel cell technologies solved
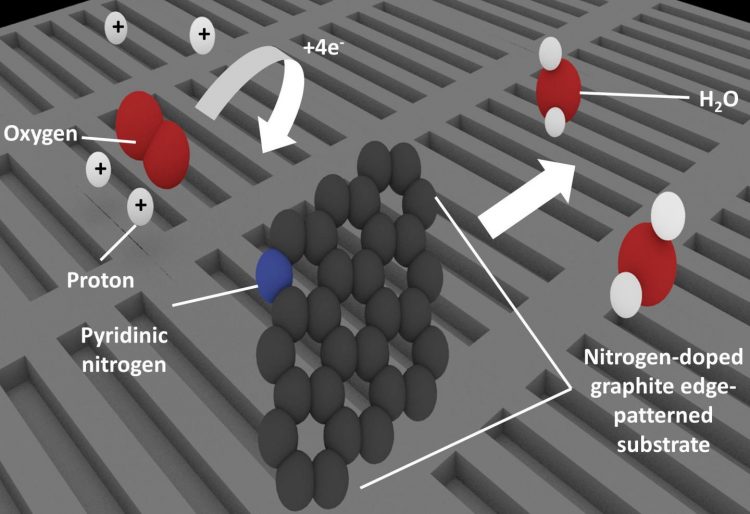
Patterning nitrogen-doped graphite to create many edges also increases the amount of pyridinic nitrogen present. Carbon atoms adjacent to pyridinic nitrogen behave as the active site for oxygen reduction, which is a key process in fuel cell technologies. Credit: University of Tsukuba
The oxygen reduction reaction is a key step in the operation of fuel cells, but depends on expensive precious metal-based catalysts. Carbon-based catalysts with added nitrogen are among the most promising alternatives to precious metals, and could allow more widespread use of fuel cell technology. However, until now, the arrangement of nitrogen and carbon that gave the catalytic effect remained a mystery, stalling efforts to develop more effective materials.
In an article published this week in Science, a team of researchers from the University of Tsukuba identified the catalytic structure and proposed a mechanism by which the reaction works. “We knew that nitrogen-doped carbon was a good oxygen reduction catalyst, but no one was sure whether the nitrogen was pyridinic or graphitic,” said corresponding author Prof. Junji Nakamura.
To solve the mystery, the team fabricated four model catalyst substrates, which simulated competing potential structures and analyzed their reaction performance. Pyridinic nitrogen, or nitrogen atoms bonded to two carbon atoms, occur mainly at the edges of the material. By patterning the substrates to change the number of edges, the team could control the presence of pyridinic nitrogen and measure how it affected the catalytic performance. These results showed that the active catalytic sites were associated with pyridinic nitrogen.
Taking the research a step further, the investigators then proposed the various stages of the reaction mechanism after finding that it was actually the carbon atom next to the nitrogen that was the active site rather than the nitrogen atom itself. As the corresponding author Prof. Nakamura noted: “Clarifying the active site and mechanism is a great step forward and will allow optimization studies to focus on driving up catalyst performance.”
Media Contact
Masataka Watanabe
kohositu@un.tsukuba.ac.jp
81-298-532-039
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



