Suitable Protein Tags for Nanoscopy
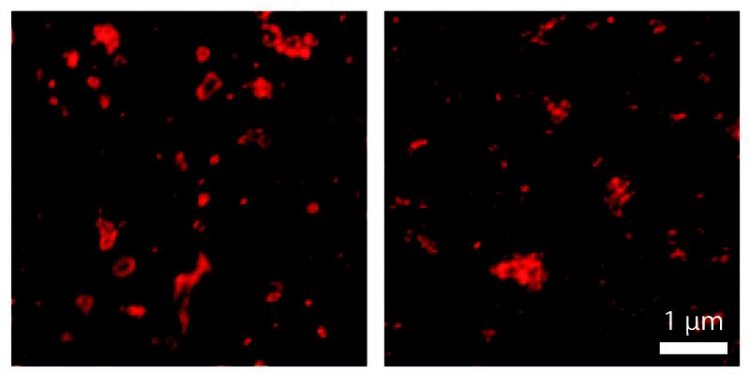
Protein labeling with aptamers (left) better displays the shape of endosomes than labeling with larger antibody probes (right). Felipe Opazo / CNMPB
Modern optical technologies such as super-resolution nanoscopy enable to exactly image small structures and molecular processes, therefore, providing a fascinating view into living cells. To visualize such processes in the nanometer range, cellular structures of interest have to be efficiently labeled.
Fluorescent proteins are routinely used as convenient tags in conventional microscopy but their use for nanoscopy has been questioned. Scientists of the Göttingen Cluster of Excellence and DFG Research Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB) and of the EMBL in Heidelberg now investigated the application of fluorescent protein tags on the organization of proteins.
The findings of the team around Nobel Prize awardee Stefan W. Hell (Max Planck Institute for Biophysical Chemistry in Göttingen), Edward Lemke (EMBL, Heidelberg) and Silvio O. Rizzoli (University Medical Center Göttingen) proved the general use of fluorescent protein tags to be reliable tools in nanoscopy and for biomedical research.
The results, recently published in ACS Nano, specially indicate fluorescence labeling based on unnatural amino acids to be a reliable alternative for labeling.
Original publication: Vreja IC, Nikić I, Göttfert F, Bates M, Kröhnert K, Outeiro TF, Hell SW, Lemke EA, Rizzoli SO (2015) Super-resolution Microscopy of Clickable Amino Acids Reveals the Effects of Fluorescent Protein tagging on Protein Assemblies. ACS NANO, 9(11): 11034-41.
Nanoscopy, or lens-based super-resolution microscopy, is a fairly new technique that enables to observe molecules and biomolecules with a resolution of 10-30 nm and to moreover analyze whole protein complexes. For their development and the implementation of super-resolution nanoscopy coauthor Stefan W. Hell, has been awarded with the Nobel Prize in Chemistry 2014 together with Betzig and William E. Moerner. For labeling and visualization procedures fluorescent protein tags are used.
Due to their relatively large size these labels often tend to form clusters, thus, producing artifacts in nanoscopic recordings. „Regarding the ease of use and their compatibility, we are specially interested in genetically encoded fluorescent protein tags, which can be introduced into living cells by gene manipulation. Their use in nanoscopy should therefore be carefully investigated“, senior author Silvio O. Rizzoli explains.
The scientists focused on 26 proteins, which are known to form various types of multi molecular arrangements and compared their nanoscale organization with or without fluorescent tag. The proteins were labeled with the smallest tag that is currently available, namely the unnatural amino acid propargyl-L-lysine (PRK) by insertion into the coding sequence of a target protein.
For visualization with a super-resolution microscope, synthetic fluorophores were coupled to the PRK via “click chemistry”. This fast and selective organic reaction is an established a standard tool for labeling and modification of biomolecules.
The researchers subsequently compared the nanoscopic organization of labeled and non-labeled proteins using ground-state depletion followed by individual molecule return microscopy (GSDIM) and stimulated emission depletion (STED) microscopy. Apart from six proteins that turned out to be slightly adversely affected, the FP-tagged and non-FP-tagged proteins formed similar molecular arrangements. In total, the scientists assessed the use of unnatural amino acids as suitable markers for nanoscopy.
“Other research groups should now also test this labeling technique to see whether their target proteins are affected by the fluorescent protein tags or not,” first author Ingrid Vreja recommends. Together with their findings, the authors suggest an easily implementable detailed protocol that other researchers may use for testing and/or labeling their own proteins of interest.
Weitere Informationen:
http://www.rizzoli-lab.de Homepage of the Department of Prof. Dr. Silvio O. Rizzoli
http://www.cnmpb.de CNMPB – Cluster of Excellence and DFG Research Center for Nanoscale Microscopy and Molecular Physiology of the Brain
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



