How photons change chemistry
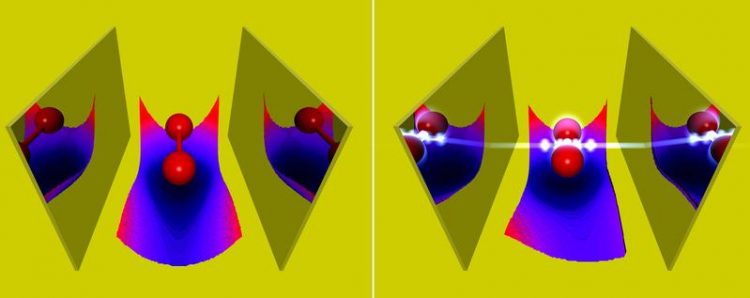
Photons in an optical cavity alter the properties of molecules, such as their binding length. Jörg M. Harms/MPSD
The chemical properties of atoms and molecules are determined by the electromagnetic interaction between the negatively charged electrons and the positively charged nuclei. In most cases the quantum nature of the interaction does not play an important role.
However, upon placing a molecule between two strongly reflecting mirrors, a so-called optical cavity, the quantum nature of the electromagnetic field can become important. In such a situation single photons can interact unusually strongly with the molecule, and one can no longer distinguish between molecule and photons. The properties of this new state of matter can be very different to the bare molecule, e.g., a higher conductivity.
Experimentally such situations have already been observed, but theoretical predictions of the chemical properties of such states were possible only to a limited extend. The reason being that the common quantum-chemical methods do not take into account the quantum nature of light.
The theory department of the Max Planck Institute for the Structure and Dynamics of Matter at CFEL has now extended some of these methods to include the coupling to the photons. Among other things, the group of Prof. Angel Rubio showed how strong coupling to photons in an optical cavity changes chemical properties of molecules, like its bond length or its absorption.
„Of special interest“, says Johannes Flick, the main author of the work, „are the changes of the Born-Oppenheimer surfaces, which are used to characterize chemical reactions. We found that strong light-matter coupling induces novel reaction pathways.“ At the same time the scientists investigated whether standard chemical reactions can be made more efficient by employing strong coupling to the photons. To do so, they considered a simple model of charge transfer between two quantum systems.
Such charge-transfer reactions are usually driven by a laser pulse. In this work, the reaction was assisted by a few photons in the optical cavity, which allowed for lower laser intensities. „Our theoretical findings do not only help to better understand the behavior of atoms and molecules strongly coupled to photons in an optical cavity,“ says Johannes Flick, „but they also highlight the possibility to change chemical properties via photons.“
In a next step the scientist want to apply their developed theoretical methods to more complex molecules. The goal is to show that the current results are generally valid and that one can alter the chemical properties of all sorts of different molecules via strong light-matter coupling.
Reference:
Atoms and Molecules in Cavities: From Weak to Strong Coupling in QED Chemistry.
Johannes Flick, Michael Ruggenthaler, Heiko Appel and Angel Rubio
PNAS, Early Edition – Doi: 10.1073/pnas.1615509114
http://www.mpsd.mpg.de/391004/20170315-flick-pnas-rubio – Institute news incl. contacts.
https://dx.doi.org/10.1073/pnas.1615509114 – Original publication site
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Innovative vortex beam technology
…unleashes ultra-secure, high-capacity data transmission. Scientists have developed a breakthrough optical technology that could dramatically enhance the capacity and security of data transmission (Fig. 1). By utilizing a new type…

Tiny dancers: Scientists synchronise bacterial motion
Researchers at TU Delft have discovered that E. coli bacteria can synchronise their movements, creating order in seemingly random biological systems. By trapping individual bacteria in micro-engineered circular cavities and…

Primary investigation on ram-rotor detonation engine
Detonation is a supersonic combustion wave, characterized by a shock wave driven by the energy release from closely coupled chemical reactions. It is a typical form of pressure gain combustion,…



