New NIST data to aid production and storage of 'fascinating' medication
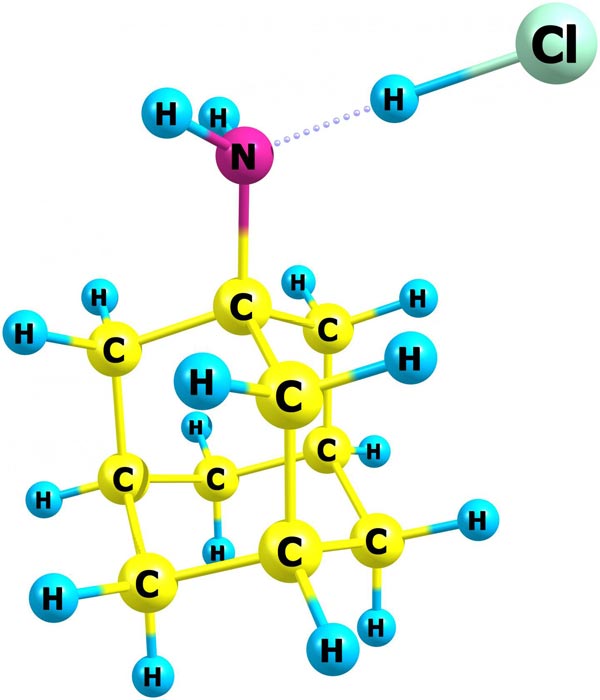
NIST chemists have published the first data on the thermodynamic properties of amantadine hydrochloride, used for many years as the active pharmaceutical ingredient for antiviral and anti-parkinsonian medications. The new information can help optimize production and storage conditions of this important compound. Its structure in the gas phase was obtained by quantum chemistry methods. In the molecular structure, C is carbon, H is hydrogen, N is nitrogen, and Cl is chlorine. Credit: Bazyleva/NIST
And yet, this compound has long been a bit of an enigma because of missing information on its properties. Now, chemists at the National Institute of Standards and Technology (NIST) and collaborators have published the very first data on this important chemical's thermodynamic properties, including data on how it responds to heat and changes from a solid into a gas.
Such data are valuable to the chemical and pharmaceutical industries for getting the highest production yields and shelf life for the medication.
“Our research results are not directly related to the medical application of this multifunctional drug, although I am really fascinated by the range of its pharmacological activity,” NIST research chemist Ala Bazyleva said.
“We studied its thermodynamic properties and decomposition,” Bazyleva said. “It is surprising, given the long history of amantadine-based drugs, that there is almost no information like this in the literature for many of them. Chemical engineers often have to rely on estimates and predictions based on similar compounds. Collating this information and developing these types of recommendations is at the core of what our group at NIST does.”
Amantadine hydrochloride belongs to a diamondoid class, a family of compounds whose structure is based on a cage of carbon atoms similar to diamond. Amantadine has a single carbon cage with a nitrogen atom attached on one side. Nonmedical studies have focused on the solid form of amantadine hydrochloride because it was expected to form disordered, or plastic, crystals, as many diamondoids do. Turns out, amantadine hydrochloride does not.
Bazyleva began studying amantadine hydrochloride years ago while in Belarus working on her doctoral dissertation, and continued the effort during her postdoctoral studies in Germany and Canada. But progress was slow, partly because adamantine hydrochloride changes from a solid directly into a gas (a process called sublimation) and simultaneously falls apart, or decomposes. She needed a model explaining this complex process, one that incorporates detailed, high-level calculations of quantum chemistry. She finally got access to this computational capability after she began working with the Thermodynamics Research Center (TRC) Group at NIST in Boulder several years ago.
“NIST was fundamental in facilitating the modeling component,” Bazyleva said. “In particular, the unique combination of facilities, software and expertise in quantum chemical computations allowed us to apply high-level calculations to get insight into the structure and stability of the drug in the gas phase.”
While the compound behaves like it is ionic (composed of positively and negatively charged pieces, though neutral overall) in the solid crystal form and when dissolved in a liquid, quantum chemistry calculations revealed that it decomposes into two neutral compounds in the gas phase.
###
The data were generated by NIST's TRC, which for more than 70 years has been producing chemical data for scientific research and industrial process design.
Co-authors are from the Belarusian State University in Belarus; the University of Rostock in Germany; and the University of Alberta in Canada.
Paper: A. Bazyleva, A.V. Blokhin, D.H. Zaitsau, G.J. Kabo, E. Paulechka, A.F. Kazakov and J.M. Shaw. Thermodynamics of the antiviral and antiparkinsonian drug amantadine hydrochloride: condensed state properties and decomposition. Journal of Chemical and Engineering Data. Published online May 1. DOI: 10.1021/acs.jced.7b00107
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….

Climate change can cause stress in herring larvae
The occurrence of multiple stressors undermines the acclimatisation strategies of juvenile herring: If larvae are exposed to several stress factors at the same time, their ability to respond to these…

Making high-yielding rice affordable and sustainable
Plant biologists show how two genes work together to trigger embryo formation in rice. Rice is a staple food crop for more than half the world’s population, but most farmers…



