Bay Area To Get Unique X-Ray Microscopy Resource
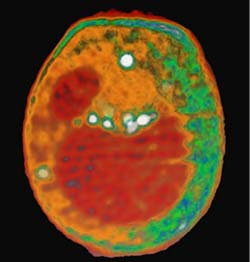
This x-ray tomography image of a yeast cell taken at the ALS with XM-1 is an example of what could be done with the proposed XM-2. Internal organelles are color-coded according to x-ray absorption. Shown in red are the nucleus (smaller sphere) and large vacuole. Lipid droplets are shown in white, and cytoplasmic structures are shown in either orange or green.
A first-of-its-kind x-ray microscope being built for the Advanced Light Source (ALS) of the Lawrence Berkeley National Laboratory (Berkeley Lab) holds forth the promise of “cat scans” for biological cells, and other unprecedented capabilities for cell and molecular biology studies. The new microscopy resource also promises a better understanding of human diseases at the molecular level and possibly new discoveries for treating those diseases. Now, researchers with Berkeley Lab and the University of California at San Francisco (UCSF), have received grants from the National Institutes of Health (NIH) and the U.S. Department of Energy (DOE) to build and operate this microscope.
“X-ray microscopy is an emerging new technology that will expand the existing imaging toolbox for cell and molecular biologists, and we would like to make this technology available to the greater biological community,” says cell biologist and microscopy expert Carolyn Larabell, who holds a joint appointment with UCSF’s Anatomy Department, and with Berkeley Lab’s Physical Biosciences Division. She is the principal investigator for this project. Berkeley Lab physicist Mark Le Gros is the co-principal investigator.
“Although there are currently many powerful techniques for imaging biological cells, each with its own unique strengths and limitations, there remains a gap between the information that can be obtained with light microscopy and electron microscopy,” Larabell says. “X-ray microscopy can bridge this gap by combining some of the best features associated with light and electron microscopy, plus bringing in some entirely new capabilities.”
To build this new microscope, Larabell and Le Gros have been awarded $2.5 million from NIH and DOE. The money will be dispersed to UCSF through NIH’s National Center for Research Resources (NCRR). Under the terms of this grant, Larabell and Le Gros will establish a Biomedical Technology Resource Center at the ALS, which means the instrument will be available to biomedical researchers throughout the nation. The new ALS x-ray microscopy resource will be the first of its kind and the 43rd such center sponsored by NCRR. Together, NIH and DOE will contribute about $1.3 million to run the microscope for each of its first five years of operation. The grants can then be renewed for additional five-year periods.
What Larabell and Le Gros have proposed is a transmission x-ray microscope (TXM) off a bend magnet beamline at the ALS, an electron-based synchrotron/storage ring capable of generating x-ray beams that are one hundred million times brighter than those from the best x-ray tubes. With beams of this intensity, the new TXM will be able to image whole, hydrated cells at resolutions of about 35 nanometers, and specific structural elements within the cell at a resolution of at least 25 nanometers. Future improvements could put the resolution of this microscope as fine as 10 nanometers, which is about the size of a protein.
Imaging data will be collected at breathtaking speed compared with the time-consuming procedures required to collect data via electron microscopy. Obtaining a complete data set for an x-ray tomography image should require less than three minutes, compared to the days and even weeks required for electron microscopy.
“We’ll be able to collect data faster than we can process it,” Larabell says. “We’ll be able to examine whole cells, identify subcellular components and locate macromolecules inside cells at substantially better resolutions than light microscopy and without the elaborate specimen preparations needed for electron microscopy.”
Microscopy using photons that fall within the visible light region of the electromagnetic spectrum remains the workhorse of biology because it enables scientists to examine living cells in their natural state. Resolution, however, is typically no better than 200 nanometers, and that is only when the light is focused on a single spot.
Microscopy techniques based on the use of electrons can provide images at a resolution of five nanometers or better. However, samples must be sliced thin and put through an elaborate chemical preparation and stained in order for the electrons to penetrate and yield high-res images.
With transmission x-ray microscopy, samples are rapidly frozen and need no further chemical alteration or staining to be imaged. Furthermore, 3-D subcelluar structures within 10 nanometers of one another can be captured in the same image, which means interactions between two or more proteins can be observed. Because of the quick turnaround time between sample preparation and data collection, scientists using the new TXM at the ALS will be able to accumulate a statistically significant volume of data within a relatively short time.
Larabell and Le Gros have been using an existing TXM at the ALS to demonstrate the potential of using this technology in cell and molecular biology studies. The existing TXM, called XM-1, the only one of its kind in the United States, was designed and is operated by the Center for X-ray Optics primarily for the study of materials. The new TXM, dubbed XM-2, will be optimized for biology and will therefore have several advantages, including improved zone plates, the optic devices composed of nanometer-scale concentric metal rings that are used to focus x-rays for imaging purposes.
“Whereas XM-1 could only be used to study suspension cell lines, XM-2 can be used to study adherent cell lines as well,” says Larabell. “In addition, we’ll have greater energy tunability with XM-2 which means it can be used to do imaging of thicker samples as well as high-res images of thinner samples just by spinning to a different zone plate.”
XM-2’s ability to use a multiple choice of x-ray beam energies will also open the door to multiple labeling of proteins. This means scientists will be able to use the microscope to study protein complexes as well as individual proteins. XM-2 should also prove to be a powerful tool for utilizing tomographic imaging techniques in x-ray microscopy. In a study using XM-1, Larabell and Le Gros performed CT scans of whole, rapidly frozen yeast cells and obtained 3-D reconstructions at a resolution of better than 50 nanometers.
“No contrast enhancement reagents were used, the cells were not embedded or sectioned, and collection of the entire data set took less than an hour,” says Larabell. “While our resolution was not quite as good as that from cryo-electron tomography, it was more than sufficient for many of the scientific questions being asked. Also, we’ve not yet reached the limit of resolution that’s theoretically possible with x-ray tomography.”
Under the terms of the NIH-NCRR grant, XM-2 is expected to be up and running at the ALS in 2006.
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



