Metallic nanoparticles will help to determine the percentage of volatile compounds
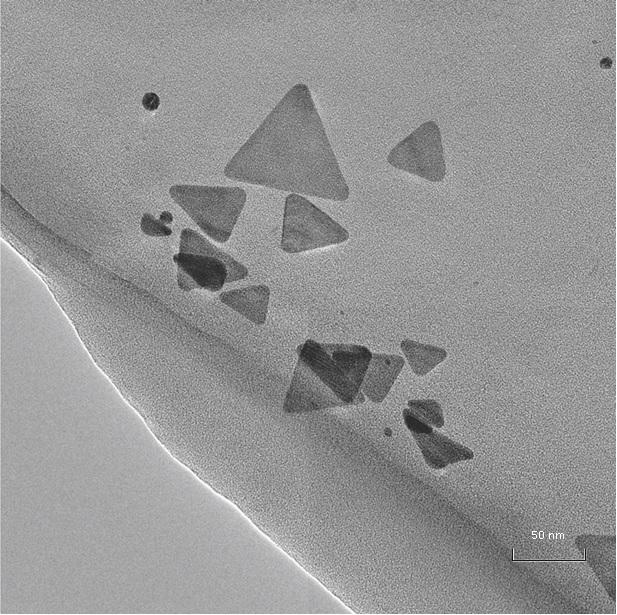
This is an image of TEM micrograph of aqueous solution of silver triangular nanoplates Credit: Aleksei Furletov
Metallic nanoparticles, in particular the nanoparticles of gold and silver, are widely used in analytical chemistry. Amongst their uses is creating optical sensors based on the surface plasmonic resonance (the phenomenon of surface plasmon excitation by light) in colloidal solutions and on solid supports.
Modern optical sensors have considerable advantages like high sensitivity, ease of detecting an analytic signal and adjustability of the optical and laboratory analysis parameters. Nevertheless, these devices have certain limitations when it comes to selectivity.
It happens because of aggregative instability of nanoparticles (the particles stick together when collide) which starts to happen during high ionic strength (high intensity of the electric field created by ions). The ion layer formed on the surface of particles is called the double electric layer and is characterized by an electrokinetic potential, also known as the zeta potential. With a decrease in the zeta potential, the electrostatic stabilization of nanoparticles does not happen.
The problem can be solved if the nanoparticles are attached to solid supports; scientists then acquire micro- or nanosensors based on solid particles. There are not many matrix materials for these sensors, and the process of attaching the nanoparticles to supports is not a simple one, so the researchers started working on a problem of modifying the surface of sensor matrices. For that goal they proposed separating the nanoparticles from ions and chemical compounds while retaining their sensitivity.
The Russian chemists invented a technique that combines optical detection using paper test strips with triangular silver nanoparticles spread over them, and dynamic gas extraction (the extraction of a compound from a solution or a dry mixture by means of liquefied gases). Perspectives of this technique were shown by detecting chlorine. Chlorine is often used to purify water, since it destroys the outer shell of bacteria and viruses. Nevertheless, the problem of determining the chlorine concentration in water remains relevant, since the existing techniques are not sensitive enough.
Aleksei Furletov, student of the Department of Analytic Chemistry, Faculty of Chemistry, Lomonosov Moscow State University, one of the paper's authors, says: “The technique developed allows to determine small amounts of gaseous chlorine in the presence of large concentrations of foreign compounds without any sample preparation. This approach can be applied to other analytical systems based on metal nanoparticles, which opens up broad opportunities for the further development of this area of chemical analysis. “
###
The research was made in collaboration with scientists from Southern Federal University, Rostov State Medical University, and Scientific-Research Institute of Chemical Reagents and Special Purity Chemicals.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



