The secret of the soybean: Mainz researchers are investigating oil bodies in soybeans
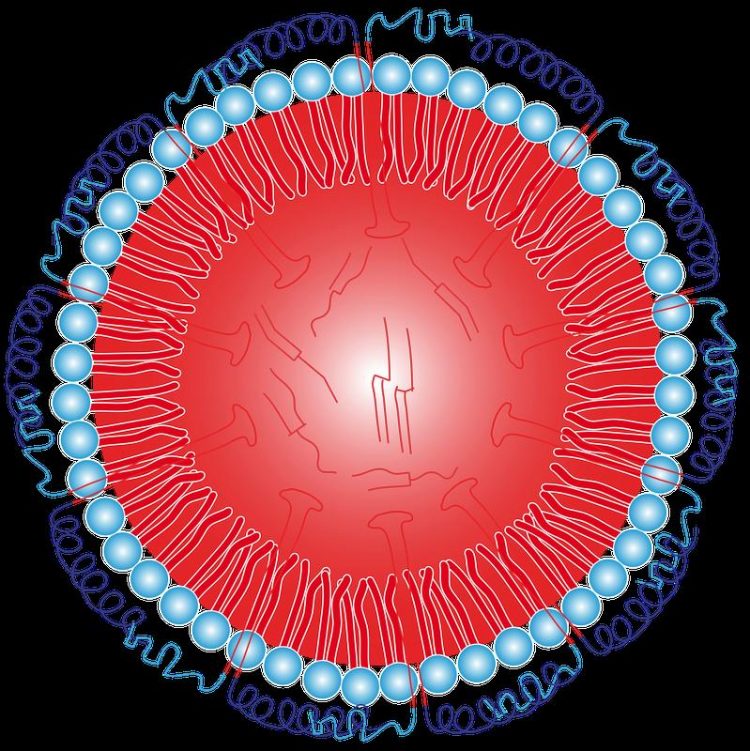
Schematic of an oleosome. Inside a shell made of phospholipids (blue) is oil. The stability is ensured by the protein "Oleosin", which penetrates deeply into the oil with an anchor. © MPI-P
Water and oil do not mix – this is an experience of everyday life. In order to mix water with oil, so-called “emulsifier agents” are needed. One of these is the molecule “Lecitin” (a phospholipid) which is also present in the soya bean.
The long-chain molecule has a water-loving as well as a water-repellent (and thus fat-loving) part. The molecule aligns itself around oil droplets and encloses them inside a sphere. The fat-loving part looks inward to the oil. Since the molecule is water-loving to the outside, small oil bodies – consisting of the emulsifier shell and the oily interior – can be dissolved in water. These oil bodies are called Oleosomes.
However, only by the presence of the emulsifier “phospholecitin”, it is not possible to explain why oleosomes are stable in the soybean plant for such a long time. “Even small temperature fluctuations and vibrations would actually destroy oleosomes – and the plant would die”, says Vilgis.
Therefore, nature creates a stability bonus through special proteins called oleosins. These oleosins penetrate deeply into the oil phase with their elongated and narrow hairpin-shaped middle part like anchors, while two water-loving arms spread protectively over the phospholipids.
In addition, these water-loving arms are electrically charged. This results in an onion-like layered structure for the only a few hundred nanometers large oleosomes which consists of the ends of the protein arms reaching into the water, the underlying phospholipids, the protein anchors penetrating into the oil and the oil core itself.
For the scientists of the “soft matter food physics” group in Mainz, these naturally occurring nanoparticles have long been the focus of research, but the exact structure of the oleosome was previously unknown. A deeper insight was possible by an accurate analysis of Small Angle Neutron Scattering-experiments. For this purpose, neutrons were shot at the nanoparticles at the research reactors in Grenoble and Oxford, and conclusions about the structure of the particles were drawn from their deflection.
This is made possible by contrast variation methods with mixtures of different concentrations of “heavy water” (whose hydrogen atoms have been replaced by deuterium) and “normal water”. The neutrons are deflected completely differently by deuterium and hydrogen, what can physically be described with the so-called “scattering length”. These lengths of the two sorts of water even have a different sign. Thus, similar to the selection of corresponding indices of refraction in optics, different layers of the oleosome can be selectively faded out and in for the neutrons. From the patterns of the scattered neutrons, the structure and size of the oil bodies can be determined.
The researchers were able to determine the diameter of the oleosome to 278 nanometers. The outer layer, the protein shell protruding into the water, is 9 nanometers thicker than previously thought. The reason for this are the positive electric charges that are present on it: because of their repulsion, only the way out in the aqueous environment of the cell and thus away from the oleosome remains. The temperature stability of the oil bodies up to 90 ° C could be directly verified by the neutron scattering measurements.
The precise knowledge of the structure of the soybean nanoparticles results in a whole series of targeted applications. Such natural nanoparticles can be used to selectively place and transport nutrients that are soluble in water and fat. While oil-soluble nutrients (e.g., vitamins) can be diffused into the interior of the particles, water-soluble substances can adhere to their surface. This is made possible by the electrically charged oleosines, whose charge can be controlled via the pH value.
The oleosomes are positive in a acidic environment, negatively charged in a basic environment. This allows the nanoparticles to be “encapsulated” in a variety of ways with biopolymers of opposing charge. This has been for example performed in the past already with pectin – a known sugar and gelling agent. Again, the layer thickness of the pectin could be measured with neutron scattering. This makes new forms of oleosome-based plant foods possible. Furthermore, the findings are not limited to soybeans, they can also be extended to the oleosomes of other oilseeds (hazelnuts, flax seeds, …). New approaches, e.g. for the geriatric nutrition can also be realized.
About Thomas Vilgis
Thomas Vilgis was born in 1955 in Oberkochen. After studying physics and mathematics in Ulm and the following doctorate, he moved to the Cavendish Laboratory in Cambridge. In 1990 he habilitated at the University of Mainz, where he was appointed professor in 1996. Since 1985 he has been research group leader at the Max Planck Institute for Polymer Research in Mainz.
In addition to his research, Thomas Vilgis is known for his numerous book publications in the field of physical and chemical aspects of food and a guest on radio and television shows. His “Journal Culinaire”, where he is the publisher, was recently named “Best of the World – 2nd Place” at the “World Cookbook Award”.
Max Planck Institute for Polymer Research
The Max Planck Institute for Polymer Research (MPI-P) ranks among the globally leading research centers in the field of polymer research since its foundation in 1984. The focus on soft materials and macromolecular materials has resulted in the worldwide unique position of the MPI-P and its research focus. Fundamental polymers research on both production and characterization as well as analysis of physical and chemical properties are conducted by scientific collaborators from all over the world. Presently over 500 people are working at the MPI-P, the vast majority of whom are engaged in scientific research.
Contact
Prof. Thomas Vilgis
Ackermannweg 10
55128 Mainz
Tel .: 06131-379 143
eMail: thomas.vilgis@mpip-mainz.mpg.de
https://www.sciencedirect.com/science/article/pii/S0021979718306015 – Original publication
http://www.mpip-mainz.mpg.de/Thomas_Vilgis/ Soft Matter Food Science Group
http://www.mpip-mainz.mpg.de Max Planck Institute for Polymer Research
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



