High-performance self-assembled catalyst for SOFC
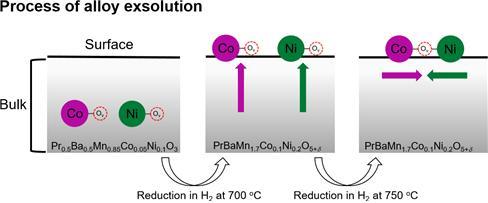
This is the process of alloy exsolution. Credit: UNIST
This breakthrough has been led by Professor Gunatae Kim in the School of Energy and Chemical Engineering at UNIST in collaboration with Professor Jeeyoung Shin of Sukmyeong Women's University, Jeong Woo Hn of Seoul University, and Professor Hu Young Jeong of UCRF at UNIST.
The new catalyst forms an alloy in which the internal material of the fuel cell rises to the surface during the operation of the fuel cell. Because of this, it does not break even if you use the hydrocarbon directly, and maintains the performance.
This study was the first to report 'the phenomenon that catalytic materials make alloys themselves to improve reaction efficiency'. The findings of this study has been selected as the front cover of the September 2018 issue of the Journal of Materials Chemistry A, as well as for the 2018 Journal of Materials Chemistry A Hot Papers.
Solid oxide fuel cells (SOFCs) have the potential to become the next major breakthrough as an alternative energy conversion device. One great appeal of SOFC is that it promises more efficient use of abundant, inexpensive natural gas, permitting less overall carbon dioxide emissions than traditional combustion turbines. They use the simple reaction of combining hydrogen and oxygen to produce electricity and water as a by-product.
One of the major challenges to developing affordable hydrogen fuel cells has been storage. This is because Hydrogen is explosive and requires costly containers to hold it safely. As a result, there has been a great increase in the development of SOFCs, using hydrocarbons, such as shale gas, natural gas, methane, propane and butane gas.
However, if the catalysts used in conventional SOFCs use hydrocarbon-based fuels, their performance will drop drastically. This is because the surface of the catalyst is contaminated with carbon or sulfur contained in the hydrocarbon-based fuel, thereby deteriorating performance. To address this, additional processes were needed to add catalyst-enhancing materials.
The research team has solved the problem with a new catalyst, designed with a layered perovskite structure. At the core of this research is to build a bi-layer perovskite structure (cobalt, nickel) that helps the chemical reactions necessary for electrical production, and when the fuel cell operates, it forms itself by itself.
“Cobalt and nickel are known to be effective catalytic materials for the operation of SOFCs,” says Ohhun Kwon in the Combined M.S./Ph.D. of Energy and Chemical Engineering at UNIST, the first author of this study. “Previously, these materials were added to make the electrodes, while the new catalysts remained in performance as they formed a “cobalt-nickel alloy.”
In fact, the catalysts developed by the researchers used methane gas directly as a fuel and operated stably with no current drop for more than 500 hours. It is also confirmed that the reaction efficiency of the catalyst is four times higher than that of the previously reported catalyst.
“The existing SOFC anode material (catalyst) was not able to operate reliably for a long time even though it showed high performance initially when using hydrocarbon fuel directly,” says Professor Kim who led the entire research. The newly developed metal alloy catalyst has excellent catalytic performance Which will greatly contribute to the popularization of fuel cells. “
###
The findings of this study have been supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP)
Journal Reference
Ohhun Kwon, et al., “Self-assembled alloy nanoparticles in a layered double perovskite as a fuel oxidation catalyst for solid oxide fuel cells”, Journal of Materials Chemistry A, (2018).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



