Quantum entanglement in chemical reactions? Now there's a way to find out
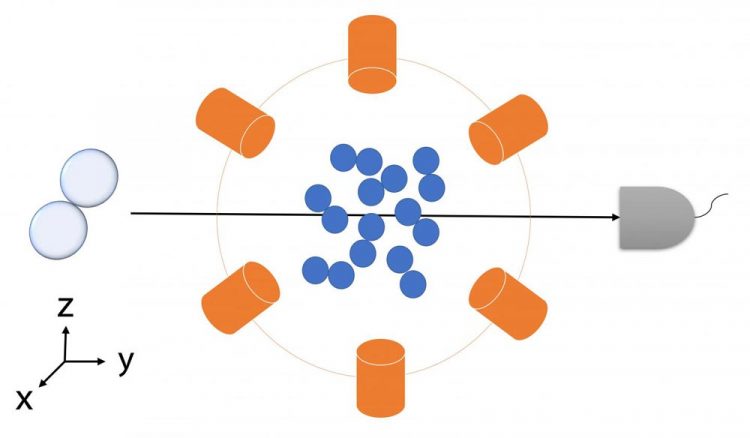
Purdue researchers have modified a popular theorem for identifying quantum entanglement and applied it to chemical reactions. This quantum simulation of a chemical reaction yielding deuterium hydride validated the new method. Credit: Purdue University image/Junxu Li
Purdue University researchers have demonstrated a new way to measure the phenomenon of entanglement in chemical reactions – the ability of quantum particles to maintain a special correlation with each other over a large distance.
Uncovering exactly how chemical reactions work could bring ways to mimic or recreate them in new technologies, such as for designing better solar energy systems.
The study, published on Friday (Aug. 2) in Science Advances, generalizes a popular theorem called “Bell's inequality” to identify entanglement in chemical reactions. In addition to theoretical arguments, the researchers also validated the generalized inequality through a quantum simulation.
“No one has experimentally shown entanglement in chemical reactions yet because we haven't had a way to measure it. For the first time, we have a practical way to measure it,” said Sabre Kais, a professor of chemistry at Purdue. “The question now is, can we use entanglement to our advantage to predict and control the outcome of chemical reactions?”
Since 1964, Bell's inequality has been widely validated and serves as a go-to test for identifying entanglement that can be described with discrete measurements, such as measuring the orientation of the spin of a quantum particle and then determining if that measurement is correlated with another particle's spin. If a system violates the inequality, then entanglement exists.
But describing entanglement in chemical reactions requires continuous measurements, such as the various angles of beams that scatter the reactants and force them to contact and transform into products. How the inputs are prepared determines the outputs of a chemical reaction.
Kais' team generalized Bell's inequality to include continuous measurements in chemical reactions. Previously, the theorem had been generalized to continuous measurements in photonic systems.
The team tested the generalized Bell's inequality in a quantum simulation of a chemical reaction yielding the molecule deuterium hydride, building off of an experiment by Stanford University researchers that aimed to study the quantum states of molecular interactions, published in 2018 in Nature Chemistry.
Because the simulations validated the Bells's theorem and showed that entanglement can be classified in chemical reactions, Kais' team proposes to further test the method on deuterium hydride in an experiment.
“We don't yet know what outputs we can control by taking advantage of entanglement in a chemical reaction – just that these outputs will be different,” Kais said. “Making entanglement measurable in these systems is an important first step.”
###
The study is based on work supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under award number DE-SC0019215.
ABSTRACT
Entanglement Classifier in Chemical Reactions
Junxu Li, Sabre Kais
Purdue University
Ever since the appearance of the seminal work of Einstein, Podolsky, and Rosen (the EPR paradox), the phenomenon of entanglement, which features the essential difference between classical and quantum physics, has received wide theoretical and experimental attentions. Recently, the desire to understand and create quantum entanglement between particles such as spins, photons, atoms, and molecules is fueled by the development of quantum teleportation, quantum communication, quantum cryptography, and quantum computation. Although most of the work has focused on showing that entanglement violates the famous Bell's inequality and its generalization for discrete measurements, few recent attempts focus on continuous measurement results. Here, we have developed a general practical inequality to test entanglement for continuous measurement results, particularly scattering of chemical reactions. After we explain how to implement this new inequality to classify entanglement in scattering experiments, we propose a specific chemical reaction to test the violation of this inequality. The method is general and could be used to classify entanglement for continuous measurement results.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A ‘language’ for ML models to predict nanopore properties
A large number of 2D materials like graphene can have nanopores – small holes formed by missing atoms through which foreign substances can pass. The properties of these nanopores dictate many…

Clinically validated, wearable ultrasound patch
… for continuous blood pressure monitoring. A team of researchers at the University of California San Diego has developed a new and improved wearable ultrasound patch for continuous and noninvasive…

A new puzzle piece for string theory research
Dr. Ksenia Fedosova from the Cluster of Excellence Mathematics Münster, along with an international research team, has proven a conjecture in string theory that physicists had proposed regarding certain equations….



