Viruses on glaciers highlight evolutionary mechanism
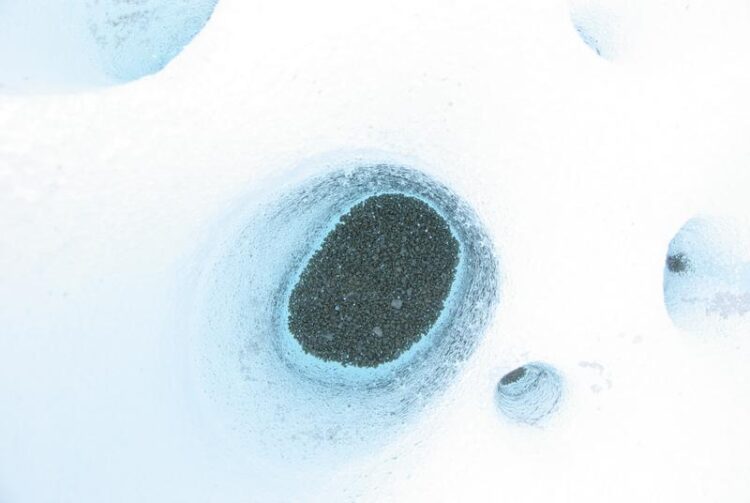
The viruses studied by the research team originate in very unusual habitats on the surface of glaciers and ice sheets, called cryoconite holes.
Gary Barker
An international team of scientists led by Christopher Bellas from the University of Innsbruck, Austria, studying life on the surface of glaciers in the Arctic and Alps challenge assumptions on virus evolution. Their study, now published in the journal Nature Communications shows that, contrary to expectations, the viruses on glaciers in the Alps, Greenland and Spitsbergen are remarkably stable in the environment.
Viruses are often thought of as a human problem, however they are the most abundant biological entities on the planet. There are millions of viruses in every teaspoon of river, lake or seawater, they are found everywhere there is life and probably infect all living organisms. Most are completely harmless to humans and infect microscopic animals, plants and bacteria, which they hijack and reprogram to produce new virus particles, most often destroying these cells in the process.
Every day, viruses destroy huge number of microorganisms in the environment, which changes the flow of energy in food webs on global scales. “Understanding how viruses evolve and function allows us to predict their role in the environment and how they interact with their hosts”, says Christopher Bellas from the Department of Ecology at the University of Innsbruck.
Together with colleagues from the Universities of Bristol, Reading and Aberystwyth in the UK, the University of Minnesota, USA, and Aarhus University in Denmark, he has sequenced and compared genomes (their total DNA) of viruses which infect microbes found on the surface of glaciers. The study, now published in the journal Nature Communications, shows that the viruses on glaciers in the Alps, Greenland and Spitsbergen have genomes which are nearly identical between these isolated locations, this contradicts what we know about the rapid evolution of viruses.
Evolutionary arms race
It is known from laboratory studies that viruses evolve rapidly in order to keep up with their hosts, which are also simultaneously evolving defenses against virus infection, this evolutionary arms race means that they should remain in balance relative to each other. This is known as the ‘Red Queen’ hypothesis after the character from Alice in Wonderland who states: It takes all the running you can do, to keep in the same place.
“This means that when we sequence the genomes of viruses from two, long-term, isolated places, we should never find exactly the same virus genomes twice”, says Christopher Bellas. The viruses studied by the research team originate in very unusual habitats on the surface of glaciers and ice sheets, called cryoconite holes. These small pools of meltwater on glaciers are ideal places to test how viruses evolve because they are miniature, replicated communities of microbes which are found on widely separated glaciers around the world.
A genetic fruit machine
When the researchers looked at the virus genomes from isolated cryoconite holes, thousands of kilometers apart, they expected to find that they would each contain different viruses only distantly related to one another. What they actually found was that most bacterial infecting viruses (bacteriophages) were nearly identical between the Arctic and the Alps. However, when they looked closer at their stable genomes, they saw that there were many small sections in each genome where DNA from other related viruses was repeatedly swapped in and out, in a known process called recombination. In each different location, the viruses shuffled the genes present in these swappable regions like a kind of genetic fruit machine.
“This means that in the natural environment, gene swapping between viruses by recombination creates much diversity in the virus population, specifically in genes which are involved in recognising and attaching to different hosts, this probably gives viruses the potential to quickly adapt to different hosts in the environment”, explains Christopher Bellas.
Wissenschaftliche Ansprechpartner:
Christopher Bellas
Department of Ecology
University of Innsbruck
mobile: +44 7808 581503
email: christopher.bellas@uibk.ac.at
web: https://www.uibk.ac.at/ecology/
Originalpublikation:
Flexible genes establish widespread bacteriophage pan-genomes in cryoconite hole ecosystems. Christopher M. Bellas, Declan Schroeder, Arwyn Edwards, Gary Barker & Alexandre M. Anesio. Nature Communications 2020. DOI: 10.1038/s41467-020-18236-8 (https://doi.org/10.1038/s41467-020-18236-8)
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

You are What You Eat—Stanford Study Links Fiber to Anti-Cancer Gene Modulation
The Fiber Gap: A Growing Concern in American Diets Fiber is well known to be an important part of a healthy diet, yet less than 10% of Americans eat the minimum recommended…

Trust Your Gut—RNA-Protein Discovery for Better Immunity
HIRI researchers uncover control mechanisms of polysaccharide utilization in Bacteroides thetaiotaomicron. Researchers at the Helmholtz Institute for RNA-based Infection Research (HIRI) and the Julius-Maximilians-Universität (JMU) in Würzburg have identified a…

ASXL1 Mutation: The Hidden Trigger Behind Blood Cancers and Inflammation
Scientists show how a mutated gene harms red and white blood cells. LA JOLLA, CA—Scientists at La Jolla Institute for Immunology (LJI) have discovered how a mutated gene kicks off…



