‘Nanojars’ capture dissolved carbon dioxide, toxic ions from water
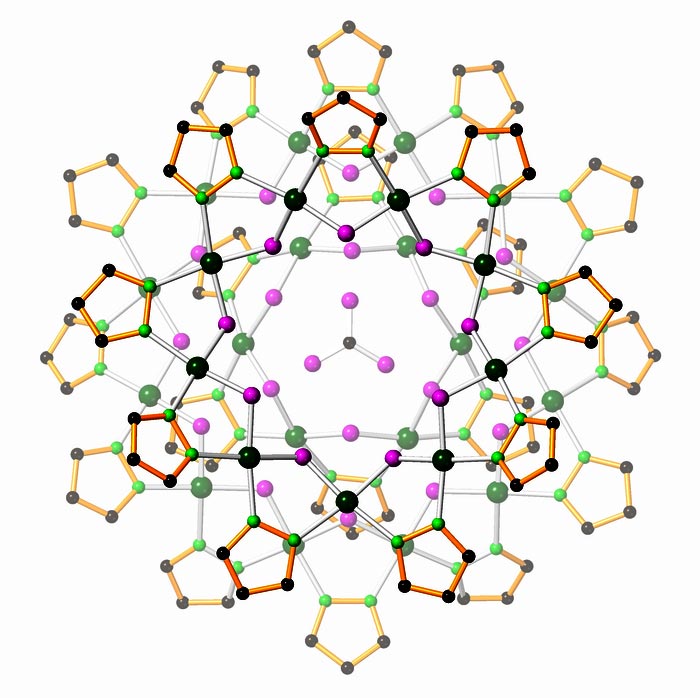
A nanojar traps a carbonate ion (center) to remove it from water.
Credit: Gellert Mezei
Carbon dioxide from the atmosphere can dissolve in oceans, lakes and ponds, forming bicarbonate ions and other compounds that change water chemistry, with possible harmful effects on aquatic organisms. In addition, bicarbonate can reenter the atmosphere as carbon dioxide later, contributing to climate change. Now, researchers have developed tiny “nanojars,” much smaller than the width of a human hair, that split bicarbonate into carbonate and capture it, as well as certain toxic anions, so the ions can be removed and potentially recycled.
The researchers will present their results today at the fall meeting of the American Chemical Society (ACS). ACS Fall 2021 is a hybrid meeting being held virtually and in-person Aug. 22-26, and on-demand content will be available Aug. 30-Sept. 30. The meeting features more than 7,000 presentations on a wide range of science topics.
“We originally developed nanojars to extract harmful negatively charged ions, like chromate and arsenate, from water,” says Gellert Mezei, Ph.D., who is presenting the work at the meeting. “But it turns out that they also bind strongly to carbonate.” Carbonate or other ions captured in the nanojars could later be disposed of or recycled into useful products, he says.
Nanojars are tiny containers made up of multiple repeating units of a copper ion, a pyrazole group and a hydroxide. The jars only form when an ion with a –2 charge, such as chromate, arsenate, phosphate or carbonate, is present. When the proper ingredients are added to an organic solvent, the repeating units form and assemble into nanojars, with the –2 charged anion bound tightly at the center.
To remove anions from water, the researchers added the solvent containing the nanojar components, which formed an organic layer on top of the water. “The solvent doesn’t mix with the water, but the anions from the water can enter this organic layer,” explains Mezei, who is at Western Michigan University. “Then, the nanojars form and wrap around the ions, trapping them in the organic phase.” Because the water and organic layers don’t mix, they can easily be separated. Treating the organic layer with a weak acid causes the nanojars to fall apart, releasing the anions for disposal or recycling.
The researchers have used nanojars to remove toxic anions from water. “We’ve shown that we can extract chromate and arsenate to below U.S. Environmental Protection Agency-permitted levels for drinking water –– really, really low levels,” Mezei says. The nanojars have an even higher affinity for carbonate, and adding a molecule called 1,10-phenanthroline to the mixture produces nanojars that bind two carbonate ions each instead of one.
The team has also made nanojars that are selective for certain anions. “The original pyrazole building block makes nanojars that are totally selective for –2 charged ions, but they can’t discriminate among these ions,” Mezei says. By using two pyrazoles tethered by an ethylene linker as a building block, the researchers made nanojars that bind preferentially to carbonate. More recently, they’ve shown that using two pyrazoles with a propylene linker produces sulfate-selective nanojars. These anion-selective nanojars will be important for applications in which only certain –2 charged ions should be removed.
The researchers have also been working on making the process more suitable for real-world applications. For example, they’ve swapped a weak base, trioctylamine, for the strong base, sodium hydroxide, originally used to make nanojars. “Trioctylamine, unlike sodium hydroxide, is soluble in the organic phase and makes the formation of the nanojars much more efficient,” Mezei says. Interestingly, trioctylamine causes nanojars to form with slightly different structures, which he refers to as “capped” nanojars, but they appear to bind carbonate just as tightly.
So far, all of the experiments have been conducted at the laboratory scale. Developing a system to treat large volumes of water, such as in a lake, will require collaboration with engineers, Mezei says. However, he envisions that contaminated lake water could be pumped into a station for treatment and then returned to the lake. Some ions, such as phosphate, could be recycled for useful purposes, such as fertilizer. Carbonate might be recycled to make “green” solvents, called carbonate esters, for the nanojar extraction itself. “Whether this process for removing carbon dioxide from water –– and indirectly, the atmosphere –– would be competitive with other technologies, that I don’t know yet,” Mezei says. “There are many aspects that have to be taken into account, and that’s a tricky business.”
A recorded media briefing on this topic will be posted Wednesday, Aug. 25 at 9 a.m. Eastern time at www.acs.org/acsfall2021briefings.
The researchers acknowledge support and funding from the National Science Foundation and Western Michigan University.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note to journalists: Please report that this research was presented at a meeting of the American Chemical Society.
Follow us: Twitter | Facebook | LinkedIn | Instagram
Title
Atmospheric CO2 sequestration by binding one or two CO32− ions in nanojars
Abstract
Nanojars are a class of neutral, nanosized toroidal copper(II)-hydroxide/pyrazolate assemblies comprised of a series of homologous oligomerization isomers of the formula [anionÌ{CuII(μ-OH)(μ-pz)}n] (Cun; n = 26−36; pz = pyrazolate, C3H3N2–), which strongly bind hydrophilic anions such as carbonate, sulfate and phosphate. A combination of single-crystal X-ray crystallography, electrospray-ionization mass spectrometry (ESI-MS) and H-NMR spectroscopy demonstrated that the various Cun nanojars are all based on a combination of three or four [Cu(OH)(pz)]x rings (x = 6−14, except 11), tightly held together by a network of hydrogen bonds and axial Cu–O interactions. The incarcerated anion guest plays a crucial role in the formation of the nanojar host, as the empty nanojar lacking a central anion cannot be obtained. Apparently, the anion is sequestered in-situ during the formation of the host-guest complex by self-assembly.
We have demonstrated that nanojars are able to sequester CO2 directly from the atmosphere by conversion into CO32- and incarceration of the carbonate ion in the center of the complex. Moreover, a second CO32- ion is bound if 1,10-phenanthroline is added to the reaction mixture. The resulting capped-nanojars, [{Cu3(μ3-OH)(μ-pz)3}CO3Ì{Cu(μ-OH)(μ-pz)}27] (1) and [{Cu2(phen)2CO3}CO3Ì{Cu(μ-OH)(μ-pz)}27] (2) have been characterized by single-crystal X-ray crystallography and electrospray-ionization mass spectrometry (ESI-MS). Complex 1 provides a new intermediate in the pH-dependent, step-wise transformation of Cu2+, OH– and pyrazolate ions into [CO3Ì{CuII(μ-OH)(μ-pz)}n]2- nanojars. The mechanism of this transformation, as well as the reversible binding of CO2 by nanojars will also be discussed.
Media Contacts
Katie Cottingham
k_cottingham@acs.org
Office: 301-775-8455
ACS Newsroom
newsroom@acs.org
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



