Shape of virus may determine RSV infection outcomes
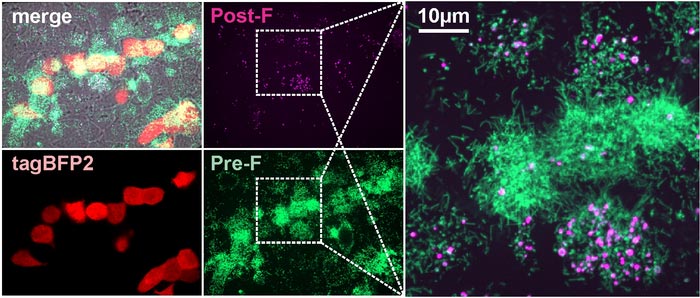
Pre-F and post-F containing RSV particles occur naturally in cell culture.
Credit: Vahey lab
Vahey’s lab investigates shape-shifting protein in common respiratory virus.
Respiratory syncytial virus, more commonly known as RSV, is a highly contagious respiratory virus that can be very serious and even fatal for young children and the elderly. In summer of 2021, health-care providers saw an unseasonable spike in the virus, which typically causes illness from October through March. While the virus has been recognized since the 1950s, there is no vaccine available.
Michael D. Vahey, a biomedical engineer at the McKelvey School of Engineering at Washington University in St. Louis, along with Jessica Kuppan and Margaret Mitrovich, both doctoral students in his lab and co-first authors of the paper, has developed a fluorescent system allowing him and his lab members to monitor the interactions between the virus particles and the proteins in the immune system that help to defend against infections, known as complement proteins. Through this system, they found that the viruses produced during RSV infection change shape — converting from long, rod-shaped particles to more rounded ones — and this makes a difference in whether the complement proteins are activated or not. Results of the work are published online in the journal eLife.
For RSV viruses to infect a cell, the membranes surrounding each of them have to fuse together through the action of the RSV F protein. Antibodies that bind to the F protein can prevent the virus from infecting cells. But the F protein is a well-known shape shifter, making it notoriously unstable, said Vahey, assistant professor of biomedical engineering, who studies infectious diseases.
“Ideally, we would like the immune system to target the form of F that can cause infection, but this doesn’t always happen,” Vahey said. “We found that when RSV changes shape from rods to spheres, the F protein tends to change shape, too. What we end up with is a situation where the virus particles that activate the complement system frequently have the wrong form of F on their surface. This may instruct the immune system to go after the wrong target.”
Vahey said the findings show that the biophysical properties of a virus, including its size and shape, matter in terms of how it is recognized by the immune system and may be important to consider when developing potential treatments or vaccines.
Journal: eLife
DOI: 10.7554/eLife.70575
Method of Research: Experimental study
Article Title: A morphological transformation in respiratory syncytial virus leads to enhanced complement deposition
Article Publication Date: 29-Sep-2021
Media Contact
Brandie Jefferson
Washington University in St. Louis
brandie.jefferson@wustl.edu
Office: 314-935-5272
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



