Researchers provide new insights into photosynthesis
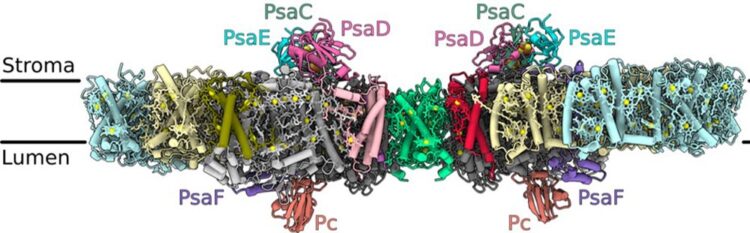
The structure of the photosystem I dimer embedded in the thylakoid membranes within the chloroplasts, where the light-driven photosynthesis process takes place (inside of the membrane: lumen, outside: stroma). The letter combinations denote different proteins that the researchers have identified.
© Nature Plants/10.1038/s41477-022-01253-4
Photosystem I in plants shows a hitherto unknown face / Molecular examination with maximum precision.
Researchers from Münster and Stockholm show for the first time that photosystem I in plants can also occur as dimers. They have examined this protein complex in a previously unseen degree of precision.
Photosynthesis is the most important basis of life on Earth. In it, plants and single-cell algae use the energy of sunlight and convert this energy into sugar and biomass. In this process, oxygen is released. Plant biotechnologists and structural biologists from the Universities of Münster and Stockholm (Sweden) have clarified the structure of a new protein complex which catalyses energy conversion processes in photosynthesis. This protein complex is the photosystem I, which is known as a single protein complex (monomer) in plants. The team of researchers headed by Prof. Michael Hippler from the University of Münster and Prof. Alexey Amunts from the University of Stockholm has now shown, for the first time, that two photosystem I monomers in plants can join together as a dimer, and they describe the molecular structure of this new kind of molecular machine.
The results, which have been published in the “Nature Plants” journal, provide molecular insights into the photosynthesis process to a hitherto unparalleled degree of precision. They could help to utilise more efficiently in future the reductive force (i.e. the preparedness to give up electrons) of photosystem I, for example to produce hydrogen as a source of energy.
The background: There are two photosynthesis complexes, called photosystems I and II, which work at their best in the case of light with different wavelengths. The uptake of light energy into photosystems I and II enables electrons to be transported within the molecular “photosynthetic machine”, thus driving the conversion of light energy into chemical energy.
In the process, electrons from photosystem I are transmitted to the protein ferredoxin. In green algae, ferredoxin can transmit electrons arising during photosynthesis to an enzyme called hydrogenase, which then produces molecular hydrogen. This molecular hydrogen is thus produced by the input of light energy, which means it is produced renewably and might be able to act as a future source of energy. The researchers asked themselves the question: “How does the production of photosynthetic hydrogen relate to the structural dynamics of the monomer and dimer photosystem I?
The results in detail
The photosystem I homodimer from the green alga Chlamydomonas reinhardtii consists of 40 protein subunits with 118 transmembrane helices providing a structure for 568 photosynthesis pigments. Using cryogenic electron microscopy, the researchers showed that the absence of subunits with the designation PsaH and Lhca2 leads to a head-to-head orientation of monomer photosystem I (PSI) and its associated light-harvesting proteins (LHCI). The light-harvesting protein Lhca9 is the key element providing for this dimerisation.
In the study, the researchers define the most precisely available PSI-LHCI model to a resolution of 2.3 Ångström (one Ångström corresponds to one ten-millionth of a millimetre), including the flexibly bound electron transmitter plastocyanin, and they allocate the correct identity and orientation to all pigments, as well as to 621 water molecules which influence the energy transmission pathways. In connection with the loss of a second gene (pgr5), the genetically induced down-regulation of the subunit Lhca2 results in the very efficient production of hydrogen in the double mutant. As Michael Hippler says, “The depletion of Lhca2 promotes the formation of PSI dimer, and so we suggest that the hydrogenase may favour the targeting of photosynthetic electrons from the PSI dimer, as we proposed in our earlier work. The structure of the PSI dimer enables us to make targeted genetic modifications in order to test the hypothesis of improved hydrogen production through the PSI dimer.”
Wissenschaftliche Ansprechpartner:
Prof. Michael Hippler
University of Münster
Phone: +49 251 83-24790
Mail: mhippler@uni-muenster.de
Originalpublikation:
Naschberger, A., Mosebach, L., Tobiasson, V., Kuhlgert, S., Scholz, M., Perez-Boerema, A., Ho, T.T., Vidal-Meireles, A., Takahashi, Y., Hippler, M., and Amunts, A. (2022). Algal photosystem I dimer and high resolution model of PSI:plastocyanin complex. Nature Plants; https://doi.org/10.1038/s41477-022-01253-4
Weitere Literatur:
Ho et al., 2022, 10.1093/plphys/kiac055; siehe auch PCT Patent Application No. PCT/IL2021/051282 ″PHOTOSYNTHETIC MICROALGAE AND USE THEREOF FOR HYDROGEN PRODUCTION”)
https://www.uni-muenster.de/news/view.php?cmdid=12892&lang=en
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



