First synthesis of a compound with aromatic nitrogen rings
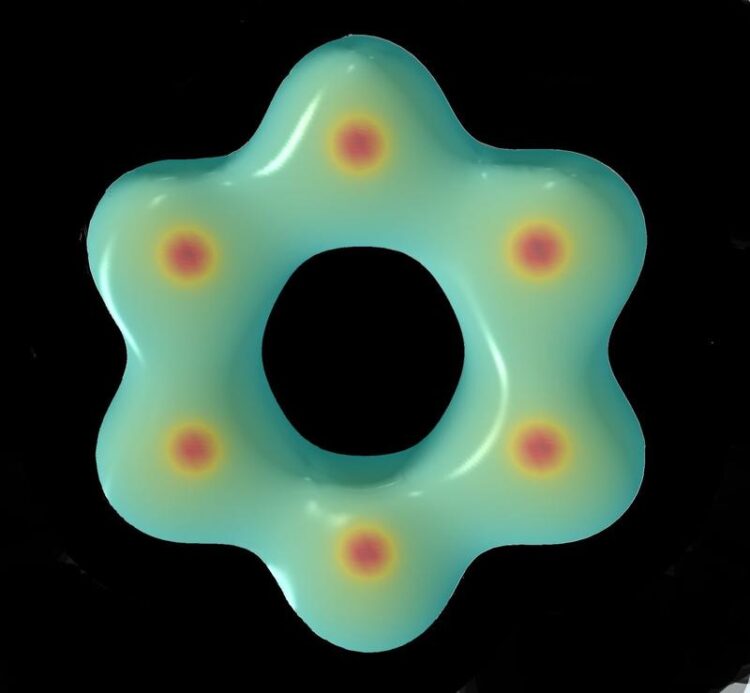
The aromatic [N₆]⁴⁻ hexazine ring.
(c) Dominique Laniel
An international team with researchers from the University of Bayreuth presents a potentially groundbreaking discovery for nitrogen chemistry in “Nature Chemistry”.
For the first time, a compound containing aromatic rings of nitrogen atoms has been synthesized. The compound of nitrogen and potassium was produced under extremely high pressures and temperatures. It has a very complex structure, but its major building block is the planar ring of six nitrogen atoms, which is called hexazine anion, as it has a negative charge. The arrangement of nitrogen atoms in the hexazine anion is similar to that of carbon atoms in benzene, an aromatic compound that is ubiquitous in nature.
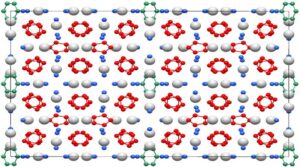
(c) Dominique Laniel
Although the term “aromatic” originally concerned odour, today its use in chemistry is restricted to compounds that have particular electronic, structural, or chemical properties. The unique stability of these compounds is referred to as aromaticity. Aromatic compounds play an important role in chemistry and biology as well as in numerous branches of industry. Although aromaticity was initially thought to be exclusive to carbon cycles, it has since been shown that numerous systems composed of carbon heterocycles and non-carbon cycles can have an aromatic character. Nitrogen aromaticity, however, has far been restricted to the [N₅]⁻ pentazolate anion.
International collaboration, involving the Laboratory of Crystallography and the Bavarian Research Institute of Experimental Geochemistry & Geophysics (BGI) at the University of Bayreuth, has now led to a considerable breakthrough. Under extreme pressure and temperature conditions, the complex compound K₉N₅₆ was synthesized, which contains [N₆]⁴⁻ hexazine rings. The structure of K₉N₅₆ is composed of a complex arrangement of [N₆]⁴⁻ and [N₅]⁻ rings as well as neutral nitrogen dimers. The researchers found that the structure of the [N₆]⁴⁻ hexazine ring conforms to the rule for aromaticity of chemical compounds named after physical chemist Erich Hückel: the ring is cyclic, planar and has (4n + 2) π-electrons (10π system).
“This is the first time that a ring consisting of six nitrogen atoms has been synthesized that follows the Hückel rule for aromaticity. Its aromatic character is further supported by bond-length considerations and by calculations of the electronic charge density. The newly synthesized [N₆]⁴⁻ hexazine ring has a striking similarity to benzene, the aromatic carbon compound which is omnipresent in nature,” says Prof. Dr. Dr. h.c. Natalia Dubrovinskaia of the Laboratory of Crystallography. “This study provides a striking example contradicting the trope of structural simplicity at high densities. We hope that this synthesis of the aromatic hexazine anion, together with that of the aromatic [N₅]⁻ pentazole anion, can stimulate further exploration of nitrogen chemistry in the search of novel nitrogen-based technological materials ,” adds Prof. Dr. Dr. h.c. Leonid Dubrovinsky of the BGI.
The complex compound K₉N₅₆ was formed under unusual conditions: Potassium azide (KN₃) and molecular nitrogen (N₂) were compressed under a pressure more than 400,000 times higher than the pressure of the Earth’s atmosphere and heated to 2,000 degrees Celsius with high-power lasers. The next step was to elucidate the internal structure of this new compound. To do this, the samples were exposed to an intense X-ray beam at two particle accelerators, the PETRA III X-ray source at the German Electron Synchrotron (DESY) in Hamburg and the European Synchrotron Radiation Facility (ESRF-EBS) in Grenoble.
“We were very surprised about the atomic arrangement of the compound K₉N₅₆: it is of a complexity almost never observed for solids produced at such high pressures. We found that it is composed of a repeating arrangement of 520 atoms: 72 K and 448 N. Although we could immediately see that the planar [N₆]⁴⁻ rings were fulfilling the basic requirements for aromaticity, we applied advanced calculations methods to verify this,” reports Dr. Dominique Laniel of the University of Edinburgh, first author of the new paper.
The necessary computational analyses were carried out by Dr. Florian Trybel and his collaborators at Linköping University. The calculations confirmed the stability of K₉N₅₆ and provided further insight into the aromaticity of the [N₆]⁴⁻ hexazine ring.
Wissenschaftliche Ansprechpartner:
Prof. Dr. Dr. h.c. Natalia Dubrovinskaia
Laboratory of Crystallography
University of Bayreuth
Phone: +49 (0)921 / 55-3880 or -3881
E-mail: Natalia.Dubrovinskaia@uni-bayreuth.de
Prof. Dr. Dr. h.c. Leonid Dubrovinsky
Bavarian Research Institute of Experimental Geochemistry & Geophysics (BGI)
University of Bayreuth
Phone: +49 (0)921 / 55-3736 or -3707
E-mail: Leonid.Dubrovinsky@uni-bayreuth.de
Originalpublikation:
Dominique Laniel et al.: Aromatic hexazine [N₆]⁴⁻ anion featured in complex structure of the high-pressure potassium nitrogen compound K₉N₅₆. Nature Chemistry (2023), WWW: https://www.nature.com/articles/s41557-023-01148-7 // DOI: https://doi.org/10.1038/s41557-023-01148-7
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

iENA Silver Medal for Observer of Atoms
In the field of precision engineering and mechatronics systems, novel innovations shape the future of technologies like nano-fabrication technology and high-precision devices. Honoring Excellence: The IMMS Patent Recently, the IMMS…

Biodegradable Plastics from Waste
In a joint research, Bioprocess engineer Prof. Dr.-Ing. Sebastian Riedel from the Berlin University of Applied Sciences (BHT) and Prof. Dr. Jaewook Myung from the Korea Advanced Institute of Science…

NASA successfully integrates Roman mission’s telescope, instruments
NASA’s Nancy Grace Roman Space Telescope team has successfully integrated the mission’s telescope and two instruments onto the instrument carrier, marking the completion of the Roman payload. Now the team…



