Growing biofilms actively alter host environment
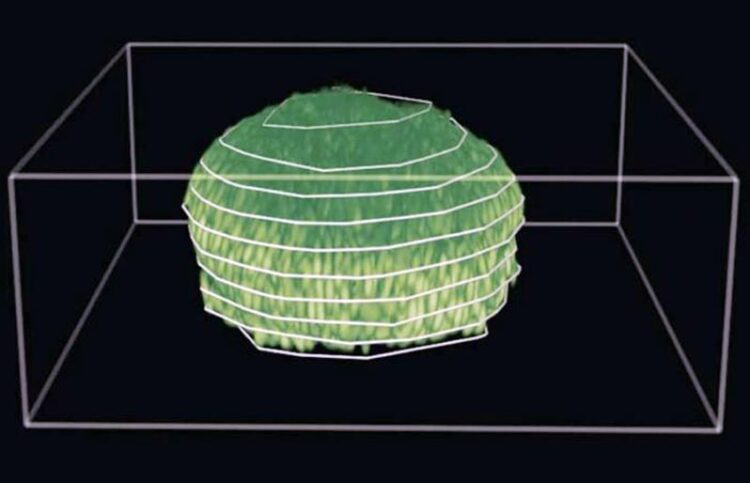
Three-dimensional reconstruction of a biofilm grown under a 0.5% agarose gel. The scale bar is 10 µm.
Credit: Provided by Jing Yan
The findings may offer insight into disease growth and the mechanics of antibiotic resistance.
Dental plaque, gut bacteria and the slippery sheen on river rocks are all examples of biofilms, organized communities of microorganisms that colonize our bodies and the world around us. A new study led by Penn State researchers reveals exactly how growing biofilms shape their environments and fine-tune their internal architecture to fit their surroundings. The findings may have implications for a wide variety of applications, from fighting disease to engineering new types of living active materials.
“In the case of bacteria, they grow, divide, and apply forces to each other and their surroundings,” said Sulin Zhang, professor of engineering science and mechanics and of biomedical engineering at Penn State and corresponding author on a paper about the discovery, recently published in the journal Nature Physics. “As such, growing bacteria have the potential to shape the environment, changing the environment they live in, so we were interested in understanding the reciprocal interactions between the growing biofilm and environment where it grows.”
Zhang collaborated with an interdisciplinary team of researchers from the Massachusetts Institute of Technology and Yale to study that interaction on all fronts: theoretically, experimentally and computationally. The researchers used biofilms made by Vibrio cholerae, which can cause cholera, as a model system to demonstrate the self-shaping and self-organizing capability of a 3D growing system.
In nature, biofilms tend to grow in tight, confined spaces, Zhang explained, so the team grew the biofilm between a soft hydrogel and a stiff glass substrate. They analyzed the growing biofilm using single-cell imaging, agent-based simulations and continuum mechanics theory. The researchers found that the biofilms shape both themselves and their boundary into an efficient formation known as “active nematics,” the arrangement of self-propelled molecules in parallel lines instead of layers.
“We found that biofilms take advantage of growth-induced stresses to shape their environment and create a nematic structure,” said Jing Yan, assistant professor of molecular, cellular and developmental biology at Yale University and co-corresponding author on the paper. “This takes us a lot closer to being able to control the morphology, the packing and ordering of the biofilm.”
Zhang explained that understanding the feedback loop between biofilm growth, growth generated stress, and its environment could pave the way for controlled growth of beneficial biofilms, the elimination of harmful ones and even the potential development of new classes of active growing materials that can respond to — and actively alter — their environment.”
Yan added that this is especially valuable information in the field of health care. Biofilms play a substantial role in disease growth in humans and animals, as they can evade the immune response. The coordinated nature of bacterial biofilms makes them highly resistant to conventional antibiotics, so they are extremely difficult to treat. In fact, the majority of chronic antibiotic resistant-infections are caused by biofilms, according to the American Society for Microbiology.
“When a bacteria enter into the body, they grow into an infection as biofilms — and they’re in a confined environment: your gut,” Yan said.
A better understanding of how biofilm-driven disease can grow in such an environment will allow researchers to develop new ways to disrupt such growth, he added.
“What we’ve learned will aid in developing strategies to tackle these infections,” said Changhao Li, a doctoral candidate in computational mechanics at Penn State and co-author on the paper. “The phenomena discovered here could lead to new strategies to suppress the growth of harmful biofilms and give us the ability to design and program beneficial ones.”
The other authors on the paper are Japinder Nijjer, Qiuting Zhang and Jung-Shen B. Tai of Yale University; Mrityunjay Kothari, Thomas Henzel and Tal Cohen of the Massachusetts Institute of Technology; and Shuang Zhou of the University of New Hampshire. The National Institutes of Health and the Charles H. Revson Foundation funded this work.
Journal: Nature Physics
DOI: 10.1038/s41567-023-02221-1
Method of Research: Experimental study
Subject of Research: Cells
Article Title: Biofilms as self-shaping growing nematics
Article Publication Date: 9-Oct-2023
Media Contact
Adrienne Berard
Penn State
akb6884@psu.edu
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



