Team of researchers in Vienna has decoded the structure of the ribonucleoprotein (RNP) of rabies virus
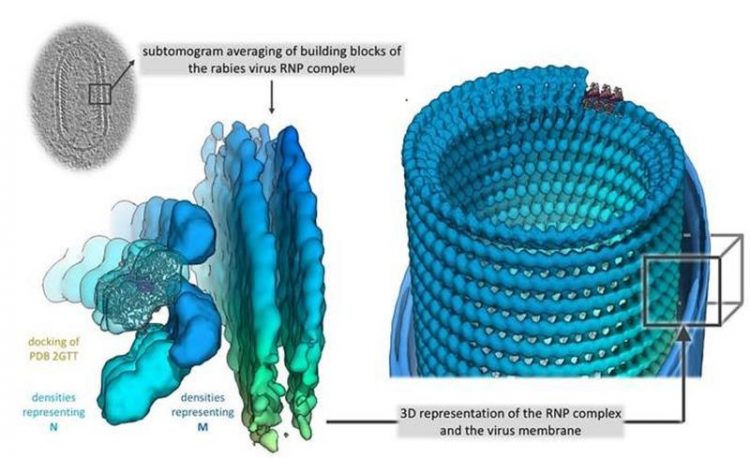
3D representation of the RNP complex © Christiane Riedel/Vetmeduni Vienna
The rabies virus (RABV) (genus Lyssavirus, family Rhabdoviridiae, order Mononegavirales) is the primary causative agent of rabies in terrestrial mammals. Human beings are also massively affected by this mortal danger.
The WHO estimates the annual human death toll to be more than 55,000. The RABV particle consists of a cell derived membrane, in which multiple copies of the surface glycoprotein are anchored, and a helical ribonucleoprotein (RNP), which forms a conical tip at one end.
Although the individual components of the RNP had already been known, the exact structure of an intact RABV-RNP complex had not yet been identified.
Using cryoelectron tomography, an imaging procedure allowing for the three-dimensional representation of the smallest biological structures, and a subsequent computer-assisted analysis by subtomogram averaging, a research team of the University of Veterinary Medicine Vienna around Christiane Riedel, the study’s first author, and Till Rümenapf, the study’s last author, has now succeeded in doing exactly that.
Two viruses as unlike siblings: similar structures, different appearances
The virus structure consists of a right-handed helix, with the 3’-terminal end of the genome located in the RNP cone, as observed in the related Vesicular stomatitis virus (VSV). Vesicular stomatitis is a virus disease with a mild course that mainly affects hoofed animals and may cause flu-like symptoms in humans.
“In RABV, interactions between M- and N-proteins are responsible for the connection of neighbouring helical turns, while M-M interactions have been described for VSV. This results in a greater distance between the helix turns compared to VSV and, thus, in a shallower angle between the individual RNP turns and the central virus axis. This shows a surprising structural variability of the RNP when comparing VSV and RABV, although the crystal structures of the individual components that had already been determined, i.e. the N- and M-proteins, are highly homologous. To put it differently: Although the individual components of RABV and VSV are very similar, there are significant differences in the architectures of the RNPs of the two viruses,” explains Christiane Riedel.
Service:
The article “Cryo EM structure of the rabies virus ribonucleoprotein complex” by Christiane Riedel, Daven Vasishtan, Vojtěch Pražák, Alexander Ghanem, Karl-Klaus Conzelmann and Till Rümenapf was published in Scientific Reports. https://www.nature.com/articles/s41598-019-46126-7
Christiane Riedel
Institute of Virology
University of Veterinary Medicine Vienna (Vetmeduni Vienna)
T +43 1 25077-2705
Christiane.Riedel@vetmeduni.ac.at
https://www.nature.com/articles/s41598-019-46126-7
https://www.vetmeduni.ac.at/en/infoservice/press-releases/press-releases-2019/te…
Media Contact
All latest news from the category: Agricultural and Forestry Science
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



