ERC Advanced Grant for cardiac research at the MDC
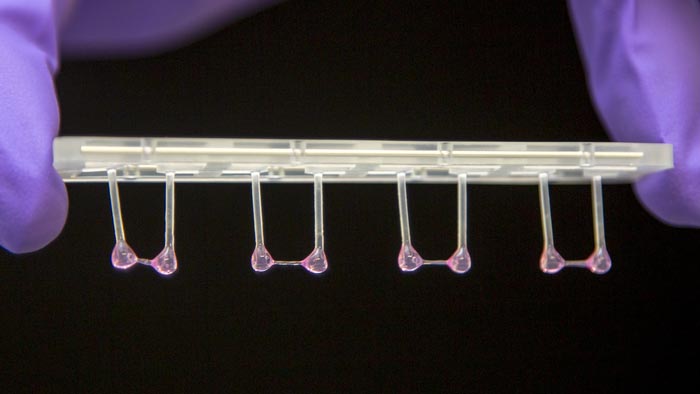
Artificial heart tissue can contract against a resistance and then relax.
Credit: Michael Gotthardt, MDC
The contractile and elastic properties of the heart are finely tuned. This is a prerequisite for the cardiac cycle and efficient adaptation. At the MDC, Michael Gotthardt investigates the underlying molecular and biomechanical mechanisms. He is awarded with an ERC Advanced Grant for this work.
MERAS is the acronym on the recently approved project proposal. It stands for: ‘Mechanoregulation of alternative splicing’. “We would like to understand how the heart responds to environmental factors and adjusts its elastic properties such that it can function at an optimal level,” says Michael Gotthardt. He heads the research group ‘Neuromuscular and Cardiovascular Cell Biology’ at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). For his project, he now receives an Advanced Grant of €2.5 million from the European Research Council (ERC).
This ERC Advanced Grant is awarded to scientists with more than ten years of research experience who have already played a prominent role in their field. Out of the 1,735 researchers from across Europe who applied for the grants this year, 253 were successful.
The mechanical work of the heart depends on the sarcomere, the smallest contractile unit. Here, actin and myosin filaments facilitate contraction, while the giant protein titin determines the elastic properties of heart muscle cells. Different titin variants (isoforms) are expressed – perfectly adapted to the mechanical needs. The researchers wish to investigate the underlying process – alternative splicing – in detail.
Refined regulatory feedback
“Our recent analysis of sarcomeric protein composition not only identified the expected structural proteins, but also proteins that link to cell signalling, metabolism, the regulation of gene expression and alternative splicing. These are proteins you would normally expect in the nucleus – but not in the sarcomere,” emphasizes Michael Gotthardt. “It appears that the sarcomere directly communicates with the nucleus on necessary adaptations.” The unexpected feedback from sarcomere to spliceosome could explain how sarcomeres adjust to mechanical stress. This is a new hypothesis that the researchers will explore in depth.
A detailed understanding of the regulatory process could also have therapeutic relevance – e.g. for people with heart failure. For most of these patients, the ventricular walls have become so rigid that the chambers are no longer able to fill sufficiently. A drug that interferes with the communication from sarcomere to spliceosome could make a stiff cardiac ventricle more compliant, resulting in more efficient filling.
A second ERC grant
This year, the ERC selection process was not solely based on the submitted proposals, but for the first time included short presentations – online of course due to Covid 19. “Four slides in eight minutes for a 2.5 million euro project,” recounts Michael Gotthardt, who is also Professor of ‘Experimental and translational cardiology’ at Charité – Universitätsmedizin Berlin. For the scientist, this is already the second substantial contribution from the EU – following his ERC Starting Grant in 2011. It extends over a period of five years. “This enables us to build up lasting collaborations and projects with extended time lines. And deep sequencing as a prerequisite to evaluate alternative splicing at scale would otherwise be prohibitively expensive.”
Gotthardt’s team works with genetic mouse models, synthetic heart tissue derived from patient cells and isolated heart muscle cells (cardiomyocytes). Single cell mechanics is precision work. “For this, the cardiomyocytes first need to be isolated, secured under a special microscope and electrically stimulated. Then you can derive the active and passive forces,” says Michael Gotthardt. This would provide the properties of just one single cell. However, for a convincing study, a large number of these experiments need to be conducted.
The goal: new technologies for single-cell mechanics and multi-omics
Extensive manual work is also required to understand which titin isoforms are expressed in response to stress or disease. Compared to a large piece of tissue, a single cell contains relatively few RNA molecules which means that analysis of gene expression frequently reaches the detection limit. “The analysis of alternative splicing is even more difficult for giant titin isoforms with up to 100,000 bases. Here, available short reads need to be assembled like a jigsaw puzzle that misses important pieces,” says Michael Gotthardt. With the ERC funding, among other things, he plans to develop technologies for single cell mechanics, -transcriptomics, -proteomics that will facilitate multi-omics approaches and enable higher rates of throughput.
The Max Delbrück Center for Molecular Medicine (MDC)
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC ), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin. www.mdc-berlin.de
Media Contact
All latest news from the category: Awards Funding
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



