Gene therapy gives long-term protection to photoreceptor cells
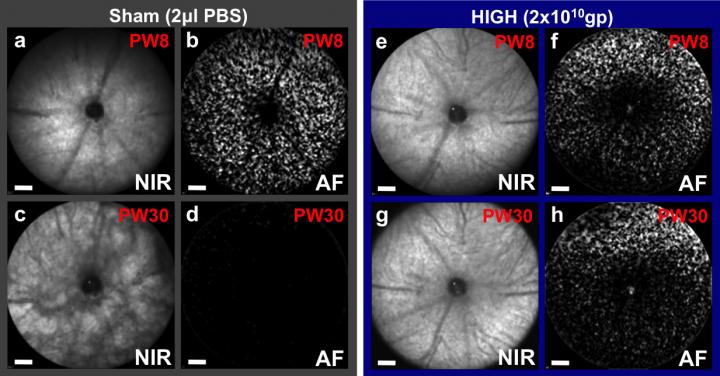
Prevention of retinal degeneration using CNTF gene therapy applied to one eye of a mouse model of retinitis pigmentosa, which also has fluorescent cones that can be counted. At 8 weeks (PW8) both eyes appear similar, but by 30 weeks retinal pigment changes can be seen in the sham injected eye (c) as the degeneration progresses with all cones lost (d). In contrast, the CNTF treated eye (g, h) has a virtually unchanged fundal appearance and over 50 percent of cones surviving (from Lipinski et al., 2015). Credit: University of Oxford/ Robert MacLaren
Results published in the journal Molecular Therapy demonstrate that the preserved cells were able to drive visually-guided behaviour, even in later stages of the condition and despite becoming less sensitive to light.
These findings are significant because they open up a new line of research to prevent nerve cell death in retinitis pigmentosa and age-related macular degeneration. They may also have a wider application to neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS).
The research was led by Professor Robert MacLaren at the University of Oxford's Nuffield Laboratory of Ophthalmology and funded in the UK primarily by Fight for Sight, with addition support from the Wellcome Trust, the Health Foundation, the Medical Research Council, the Royal College of Surgeons of Edinburgh, the Oxford Stem Cell Institute and the NIHR Ophthalmology (Moorfields) and Oxford Biomedical Research Centres.
*In detail*
Retinitis pigmentosa (RP) affects 1 in 4000 people, with symptoms that typically appear between age 10 and 30. Night vision and peripheral vision go first, as the photoreceptors active in low light – the 'rods' – start to degenerate. Eventually the condition affects the 'cones' – the photoreceptors responsible for central, detailed, colour vision.
The current study looked at a mouse model of retinitis pigmentosa in which the mice lack rhodopsin – the main pigment in rod photoreceptors. At age 4 weeks – after rod degeneration was underway and before cones were affected – the mice were dosed with a virus modified to produce human ciliary neurotrophic factor (CNTF) protein in the retina.
CNTF is a compound previously shown to prevent the loss of photoreceptors and retinal ganglion cells. However its use as a potential treatment has been in question because of concerns about toxicity. The unique approach in this study was to use a mouse which had fluorescent green cone photoreceptors which could be counted by examining the living retina with a modified ophthalmoscope at various timepoints during the course of the degeneration. This allowed precise titration of the gene therapy dose which minimised any toxic effects.
Treatment was given to one eye, while saline was given to the other eye as a control. At 8 weeks, non-invasive imaging showed similar numbers of cones in all treated and untreated eyes. However, the number of cones decreased rapidly over the time course of the experiment in low-dose and control eyes, reaching 0 by week 24.
What's really interesting is that, in contrast to previous CNTF studies, the research team was able to show that the preserved cones were functional by using behavioural tests and imaging blood flow in the visual cortex.
Next-generation sequencing at 30 weeks revealed that a group of genes previously linked to retinal disease were up to 89 times more active in high-dose eyes than in controls. Several genes usually active in the retina were also found to be less active in both medium- and high-dose eyes.
“Our results in this mouse model of retinitis pigmentosa clearly show that CNTF treatment can both give life-long protection to cone photoreceptors and preserve useful vision. While there remains a lot to understand, for example on the role of rods in cone preservation and translation to human retinal anatomy, this is a very promising study,” said MacLaren, Professor of Ophthalmology at the Nuffield Laboratory of Ophthalmology.
“We already know from clinical trials aimed at preventing motor neuron loss in ALS that high-dose systemic treatment with CNTF causes too many adverse reactions to be tolerated by patients. However, our results suggest that directly increasing activity in the class of genes that were upregulated in our high-dose CNTF group has the potential to provide a novel, targeted treatment for retinitis pigmentosa and a range of neurodegenerative diseases.”
Dr Dolores M Conroy, Director of Research at Fight for Sight, said:
“One of the most exciting prospects about these results is that the CNTF treatment was able to preserve vision even though rod degeneration had begun. This is a top priority for people with inherited and progressive eye conditions such as retinitis pigmentosa and AMD. As with any new line of research there is still a long way to go before any treatment could reach the clinic, but it is certainly possibly to look ahead and see that there will be a day when we can prevent sight loss with complex genetic involvement.”
Citation
Lipinski, D.M., Barnard, A.R., Singh, M.S., Martin, S., Lee, E., Davies, W.I.L. & MacLaren, R.E. CNTF gene therapy confers lifelong neuroprotection in a mouse model of human retinitis pigmentosa. Mol. Ther., 2015.
URL
http://palgrave.
Image URL, caption & credit
Caption: Prevention of retinal degeneration using CNTF gene therapy applied to one eye of a mouse model of retinitis pigmentosa, which also has fluorescent cones that can be counted. At 8 weeks (PW8) both eyes appear similar, but by 30 weeks retinal pigment changes can be seen in the sham injected eye (c) as the degeneration progresses with all cones lost (d). In contrast, the CNTF treated eye (g, h) has a virtually unchanged fundal appearance and over 50% of cones surviving (from Lipinski et al., 2015).
Credit: ©University of Oxford
Fast facts
* More than 190 genes are linked to inherited retinal disease of which about half cause retinitis pigmentosa (RP).
* We still don't know the exact genetic cause of photoreceptor loss in 30% of people with autosomal recessive RP (inherited from both parents) and 50% of people with autosomal dominant RP (inherited from one parent).
* Retinal ganglion cells form the optic nerve – the specialised cable that transmits visual signals from eye to brain.
* Age-related macular degeneration (AMD) is one of the leading causes of sight loss in the UK. Most of the sight loss due to AMD happens during the later stages, which affect around 0.5 million over 50s in the UK and counting.
* Genetics is an important factor in the risk of developing AMD.
Fight for Sight is the leading UK charity dedicated to funding pioneering research to prevent sight loss and treat eye disease. Fight for Sight is funding research at leading universities and hospitals throughout the UK.
Major achievements to date include: saving the sight of thousands of premature babies through understanding and controlling levels of oxygen delivery; restoring sight by establishing the UK Corneal Transplant Service enabling over 52,000 corneal transplants to take place; providing the funding for the research leading to the world's first clinical trial for choroideremia; bringing hope to children with inherited eye disease by co-funding the team responsible for the world's first gene therapy clinical trial; and identifying new genes responsible for keratoconus, Nance-Horan syndrome, achromatopsia and retinitis pigmentosa.
Fight for Sight's current research programme is focusing on preventing and treating age-related macular degeneration, diabetic retinopathy, glaucoma, cataract and corneal disease. We are also funding research into the causes of childhood blindness and a large number of rare eye diseases.
###
For more information, please contact: Ade Deane-Pratt on 020 7264 3906 or ade@fightforsight.org.uk
http://www.
The Nuffield Laboratory of Ophthalmology is part of Oxford University's Medical Sciences Division, one of the largest biomedical research centres in Europe, with over 2,500 people involved in research and more than 2,800 students. The University is rated the best in the world for medicine, and it is home to the UK's top-ranked medical school.
From the genetic and molecular basis of disease to the latest advances in neuroscience, Oxford is at the forefront of medical research. It has one of the largest clinical trial portfolios in the UK and great expertise in taking discoveries from the lab into the clinic. Partnerships with the local NHS Trusts enable patients to benefit from close links between medical research and healthcare delivery.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



