Keeping our cells stable: A closer look at microtubules
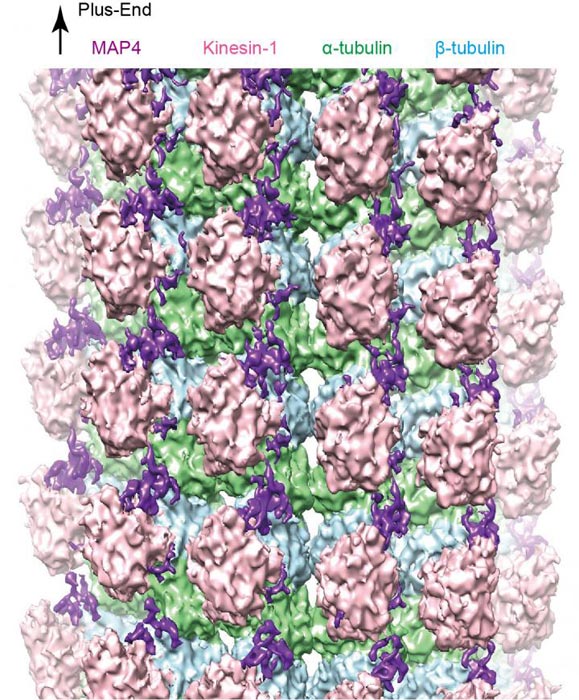
Figure 1: A cryo-EM reconstruction of the microtubule-MAP4-kinesin complex. Credit: Kobe University
The research team was led by Professor Ryo Nitta and Project Professor Tsuyoshi Imasaki (Kobe University Graduate School of Medicine) in collaboration with team leader Mikako Shirouzu and Researcher Hideki Shigematsu (RIKEN), and Associate Professor Kiyotaka Tokuraku (Muroran Institute of Technology).
Cells in our bodies take on specialized shapes in order to function as part of organs and tissue. For example, nerve cells keep the brain and body closely linked by making a communications network between cell projections. Heart cells form lines of cylinders for effective muscle contraction.
To create these shapes, a framework of complex proteins make the cell “skeletons”. The widest of these are known as microtubules, and their placement is regulated by microtubule-associated proteins.
Tau and MAP4 (both part of the Tau family) are “classic” microtubule-associated proteins. Tau is found in nerve cells, while MAP4 is expressed widely throughout our bodies such as the heart or skeletal muscle.
Excessive expression of these classic microtubule-associated proteins has been linked to Alzheimer's disease and heart failure. It can block the movement of motor protein kinesin, which uses microtubules as “rails” to transport various substances within cells.
The research team reconstructed the complex structure of microtubules, MAP4 and motor protein kinesin under laboratory conditions, and used cryo-electron microscopy to visualize the detailed three-dimensional structure (figure 1).
Their analysis revealed that MAP4 attaches to the long axes of microtubules and stabilizes them. The bonds between MAP4 and microtubules are located at two types of sites: for strong and weak interactions. At the weak sites, kinesin competes with MAP4 to bind with microtubules (see figure 2). If there is sufficient kinesin, it can displace the MAP4 at the weak sites and bind with the microtubules.
This leads to both MAP4 (at the strong anchor sites) and kinesin (at the weak sites) binding with microtubules at the same time. The team found that, as well as binding directly with microtubules, MAP4 also folds and accumulates above the microtubules.
The MAP4 in this area interacts with and secures kinesin, blocking the movement of kinesin above the microtubules. This shows how MAP4 stabilizes microtubules, and how it also blocks the transport functions of kinesin.
This research provides important information that could potentially help to create a new treatment strategy for cardiac hypertrophy and heart failure caused by overexpression of MAP4. It is also highly possible that Tau, which has an amino-acid sequence very similar to MAP4, could present the same structure. In this case, this study would also shed light on neurodegenerative diseases such as dementia.
Professor Nitta comments: “By revealing the micromorphology of the MAP4 and microtubule complex in cells, we hope this research will provide insights on a cellular level that can help us to combat diseases caused by cell change such as heart failure and dementia.”
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



