Scientists discover how gene mutation triggers immune disease
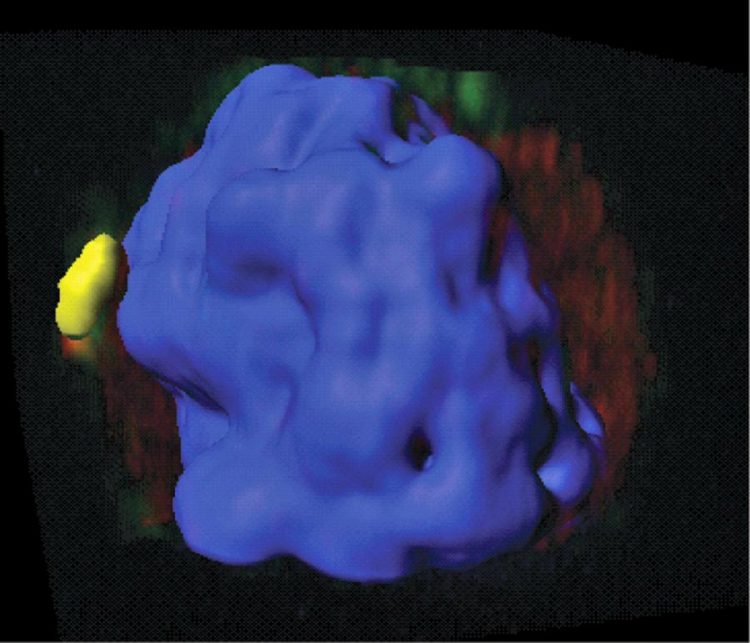
This confocal microscopic image of a healthy mouse T cell uses color florescence to illustrate how the protein Gimap5 (upper-right faint green area) and the enzyme GSK3 (red) overlap in small structures called vesicles inside cells (colocalized yellow area). This happens before GSK3 enters the cell nucleus (shown in blue). This step is important to limit DNA damage in active T cells during their expansion. The image is part of a study published Jan. 30 by Nature Communications from researchers at Cincinnati Children's. Credit: Cincinnati Children's / Publication in Nature Communications
Scientists discovered how a gene mutation affects T cell function to promote immune disorders and then tested a treatment based on the discovery–successfully fixing donated immune cells from a 16-year-old boy with an abnormally low level of white blood cells called lymphopenia.
Cincinnati Children's researchers report their findings Jan. 30 in Nature Communications. The discovery centers on mutation of the gene Gimap5, which is important to the healthy formation and function of CD4+ T cells, one of the immune system's super soldiers against infection and disease.
The protein associated with the Gimap5 gene (also Gimap5), is important because it regulates a protein that inactivates an enzyme called GSK3, researchers said. If GSK3 isn't inactivated it causes DNA damage in T cells that are expanding, causing the cells to not survive or function correctly. In mice and human blood cells, the researchers tested drugs that inhibit GSK3, improving immune system function in mice and restoring normal T cell function in the human cells.
GSK3 inhibitors already are used to treat other diseases like Alzheimer's, mood disorders and diabetes mellitus.
“Our data suggest GSK3 inhibitors will improve T cell survival and function and may prevent or correct immune-related disorders in people with Gimap5 loss-of-function mutations,” said Kasper Hoebe, PhD, Division of Immunobiology. “Therapeutically targeting this pathway may be relevant for treating people with Gimap5 mutations linked to autoimmunity in Type 1 diabetes, systemic lupus erythematosus or asthma.”
Hoebe led the study, together with Andrew Patterson, a PhD student in Hoebe's lab, and Jack Bleesing, MD, PhD, in the Division of Bone Marrow Transplantation and Immune Deficiency.
Immune system disorders lead to abnormally low immune activity (deficiency) or overactivity (autoimmunity). Immune deficiency diseases decrease the body's ability to fight infection, while autoimmunity prompts the body to attack its own tissues. Both are common causes of illness, and malfunctioning T cells are linked to both.
Translating the Science
Authors of the current study said additional research is needed before the data have clinical relevance for patients. New experiments are underway to translate the findings into the clinic, Hoebe said.
The scientists are investigating if and how genetic variants in Gimap5 affect GSK3 regulation cause malfunctioning T cells in patients with immune disorders. They also are exploring the therapeutic potential of GSK3 inhibitors in preclinical mouse models of allergic lung disease and lupus to see if they can improve patient outcomes.
“We believe the use of GSK3 inhibitors to prevent or correct these type of immune-related diseases holds great potential,” Hoebe said.
GIMAP5 Backstory
The Gimap5 gene controls its associated protein Gimap5 (GTPase of immunity associated protein 5). As the name suggests, its role is mainly linked to immune system function, lymphocyte white blood cell survival and T cell formation in the thymus.
Genetic variants in Gimap5 were already associated with autoimmunity and colitis, but their specific biological mechanisms have remained unclear. Hoebe and colleagues show Gimap5 is essential for inactivating the GSK3 enzyme (known as glycogen synthase kinase-3) when T cells are activated. Otherwise, the activity of GSK3 constrains other molecular processes and prevents T cells from completing their cell cycle to full functionality.
###
Besides Cincinnati Children's, which led the research, the study included collaborators at the Harvard T.H. Chan School of Public Health, the Cleveland Clinic and Mt. Sinai Hospital in Toronto.
Funding support came in part from the Crohn's & Colitis Foundation of America (#3793), the National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases (P30-DK078392) and the Integrative Morphology Core of the Cincinnati Digestive Disease Research Core Center at Cincinnati Children's.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



