A breakthrough in acidic water electrolysis via ruthenium-based catalysts
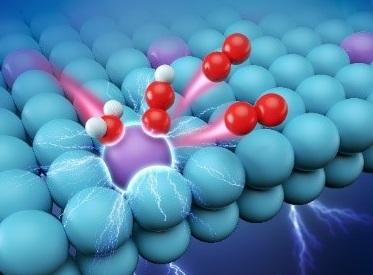
In acidic electrolyte environment, water molecules was adsorbed on the active site Ru atom, and then single atom Ru catalyst boosts acidic water electrolysis producing oxygen. Purple, blue, red and white balls represents Ru, Pt, O and H atoms, respectively. Credit: WU Yuen
Fortunately, a recent study is bringing this dream closer. Professor WU Yuen's team from the University of Science and Technology of China (USTC) successfully prepared a kind of Ruthenium (Ru) single atom alloy catalyst, which greatly accelerates the process of water eletrolysis with lower overpotential (220 mV).
By surface defect engineering to capture and stabilize single atoms, the group successfully prepared the single-atom catalyst. It is capable of delivering a 90 mV lower overpotential to reach a current density of 10 mA/cm2, and an order of magnitude with longer lifetime than that of commercial RuO2.
In this study, researchers constructed a series of alloy-supported Ru1 using different PtCu alloys through sequential acid etching and electrochemical leaching. They also found a volcano relation between oxygen evolution reaction (OER) activity and the lattice constant of the PtCu alloys.
Density functional theory investigations reveal that the compressive strain of the Pt-skin shell engineers the electronic structure of the Ru1, allowing optimized binding of oxygen species and better resistance to over-oxidation and dissolution.
Compared with Iridium-based systems, which have better dissolution resistance, Ru-based ones have more abundant reserves and have been evaluated to be a more active OER catalyst due to its lower overpotential.
This research makes hydrogen production through water electrolysis easier and more efficient, and allows people to see the great potential of hydrogen as an alternative new energy in the future.
Nevertheless, till now, the stability problem of catalyst hasn't been completely resolved. “There are still many explorations left to further improve the reaction system and we are going to continue designing experiments and trying to find the best way to boost activity and stability of catalysts.” said Ph.D. YAO.
###
The results were published on Nature Catalysis as cover story.
Media Contact
More Information:
http://dx.doi.org/10.1038/s41929-019-0246-2All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



